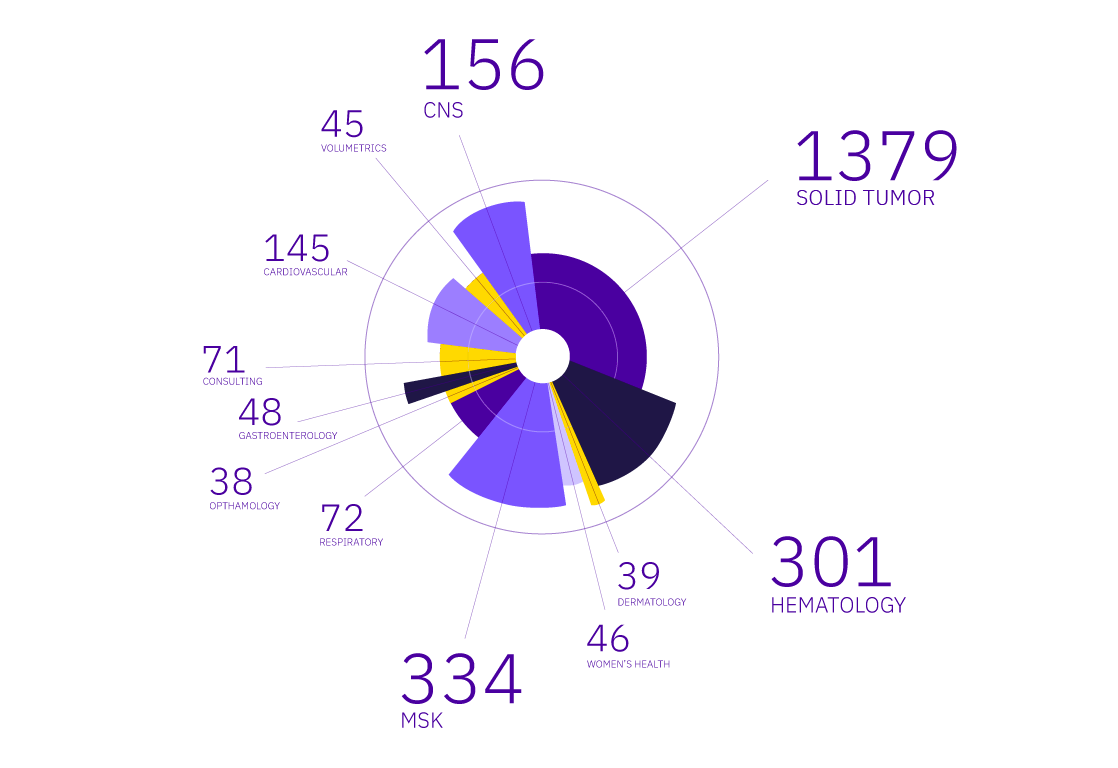
Since 1997, Calyx has helped global biopharmaceutical companies and CROs successfully leverage medical imaging in their clinical trials. We offer a full range of imaging services and deliver therapeutically aligned value that allows you to best assess the safety, efficacy, and effectiveness of your compounds, time after time.
Whether you’re in early phase development looking to partner, planning to take your compound through regulatory approval, or even seeking breakthrough therapy designation / approval, lean on Calyx’s 25+ years’ experience and benefit from a tailored core imaging strategy proven to meet your clinical development objectives.