Successful medical imaging in solid tumor trials requires the insight and expertise of professionals who are dedicated to providing imaging core lab services and have therapeutic experience, expertise in the modalities required to demonstrate safety and efficacy, and first-hand insight as to what global regulators will look for in your submissions.
Modalities, criteria, and regulators’ expectations change frequently. Without the direction of imaging scientists who work day-in and day-out in clinical trial imaging, it would be difficult, if not impossible to keep track of and react to changes prior to study start and First Patient In.
A Look at Changing Regulations in Solid Tumor Research
Your imaging partner needs to be immersed in the scientific advances and regulatory changes that may impact the outcome of your anti-tumor treatment development program, as outlined in the following examples:
EXAMPLE 1: ANTIBODY DRUG CONJUGATES (ADCS)
Advances in oncology precision medicine and therapy have increased the number of immune therapies and molecular-targeted agents for the treatment of most solid tumors. Although ADCs are one of the fastest-growing classes of cancer drugs in clinical development, they have been associated with the risk of pulmonary abnormalities, the most concerning of which is Drug-induced Interstitial Lung Disease (DI-ILD).
As a result, regulatory agencies around the world are asking trial sponsors to monitor for ILD toxicity in clinical trials investigating ADCs for the treatment of cancers. High-resolution Computed Tomography (HRCT) is the gold standard for the detection and characterization of ILD and, when combined with clinical and laboratory data, provides a powerful method for accurate diagnosis.
Integration of radiology data with clinical and laboratory tests such as pulmonary function tests and review of this information by a centralized independent joint events committee consisting of expert clinicians provides a consistent and standardized way to adjudicate DIILD cases.

Calyx’s Dr. Oliver Bohnsack leverages his knowledge from having co-authored the immune-related response criteria (irRC, 2009), served as first author of irRECIST (2014), and co-authored Comparison of Assessments using RECIST and irRECIST by Manitz J. et al. (2020) as he designs and implements optimal imaging strategies for Calyx’s customers developing new oncology treatments
EXAMPLE 2: TUMOR-INFILTRATING LYMPHOCYTE (TIL) AND CAR-T THERAPIES
Calyx has experience supporting imaging trials in cell therapy and understands the evolution of imaging findings in patients undergoing such therapeutic
intervention. This allows us to ensure appropriate guidance is documented in the imaging charter and independent readers are trained to provide an assessment in consideration of such novel therapeutic intervention.
EXAMPLE 3: IMMUNO-ONCOLOGY RESEARCH
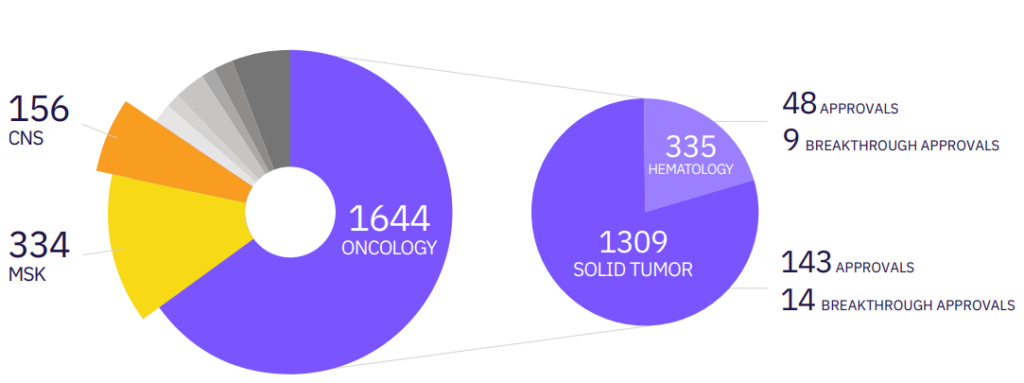
Calyx Medical Imaging scientists have the industry’s most extensive experience in oncology clinical trial imaging and are immersed in their therapeutic areas of expertise and modality specialties. The team of 80+ internal scientific and medical experts has successfully managed the imaging components of over 1,500 clinical trials across 90 unique oncology indications and has supported over 150 solid tumor regulatory approvals involving the entire span of imaging modalities utilized in tumor patient care.
In Solid Tumor Imaging, Experience Matters
Calyx Medical Imaging scientists have the industry’s most extensive experience in oncology clinical trial imaging and are immersed in their therapeutic areas of expertise and modality specialties. The team of 80+ internal scientific and medical experts has successfully managed the imaging components of over 1,500 clinical trials across 90 unique oncology indications and has supported over 150 solid tumor regulatory approvals involving the entire span of imaging modalities utilized in tumor patient care.
Calyx Medical Imaging Experience
Calyx’s scientists are committed to the success of your clinical trial, maintaining regular contact with your team throughout its duration and overseeing the important independent imaging reads that can make or break your trial. This becomes even more critical when your study-specific needs change, like when regulators question the initial data submitted or request additional information on how reads were conducted. Or, if early results demonstrate superior efficacy and earn your compound fast-track designation, significantly growing and accelerating your imaging needs, most times overnight.
The Value of a Scalable Reviewer Network and Operational Infrastructure
A key component of Calyx’s Medical Imaging offering is the delivery of accurate and precise imaging assessments performed by board-certified experts from a network of over 1,200 independent readers who are well-recognized in their fields. Calyx’s network of readers is active in the clinic and works with patients regularly, ensuring they have a comprehensive and up-to-date understanding of your patients, the disease, and the role of imaging in their assessment.
And, Calyx’s operational model enables our client delivery teams to anticipate your study needs and scale our services to deliver quick and reliable continuity of service with efficient setup of sites and reduced imaging queries to meet your trial timelines, even during mid-study changes.
Having access to so many retained, experienced radiologists – the majority of whom have worked with Calyx for over a decade – and deep operational expertise delivered through seven global locations centered in key life science hubs, Calyx Medical Imaging delivers the flexibility and scalability trial sponsors need during all stages of solid tumor treatment development, helping them to overcome challenges and tackle unexpected trial changes as evidenced in the following case studies.
Calyx Medical Imaging:
By the Numbers
450,000+
ANNUAL READS FROM GLOBAL SITES
4.6M
TOTAL IMAGES PROCESSED
<24-HR
CASE RESOLUTION (80K ANNUAL CASES)
50+
STUDIES WITH >10K TIMEPOINTS
120+
STUDIES WITH >1K PATIENTS
575+
STUDIES WITH <100 PATIENTS
Case Study: Scaling to Support Melanoma PD1/PDL1 Breakthrough Trial
A client approached Calyx Medical Imaging for support with a PD1/PDL1 trial aimed to treat patients with advanced or unresectable melanoma who were no longer responding to other drugs. The FDA granted breakthrough therapy designation based on data supported by Calyx’s central reviews which demonstrated preliminary clinical evidence that the drug potentially offered a substantial improvement over available therapies on the market.
The phase I study quickly became an advanced phase III study. Calyx supported a quick ramp-up, providing senior project management and industry-leading scientific guidance to help meet the demands of breakthrough therapy designation. This involved rapid modifications to the imaging charter and read design, adjusting from single review to double review with adjudication and clinical data evaluation by a central oncologist.
Calyx immediately expanded its reader pool and contracted nine additional radiologists and oncologists with expertise in melanoma imaging to meet the sponsor’s expedited review timelines and increased read volume.
Calyx’s medical imaging expertise and access to a deep network of experienced independent readers proved to be a key contributor to the success of the trial and to the FDA’s accelerated approval of the novel melanoma treatment.
Case Study: Meeting FDA Request for Expanded Imaging During RCC Compound Development
Calyx supported a phase III Renal Cell Carcinoma adjuvant therapy trial with approximately 1,000 enrolled patients. The trial began with an audit methodology reading only 20% of subjects and later, after receiving FDA feedback, expanded to a full review of all subjects.
The sponsor was interested in obtaining the full set of data as quickly as possible. Calyx responded by adding eight experienced readers to the study’s reviewer pool and managed expedited review for all cases. The Calyx study team closely monitored reader performance as data was reviewed, ensuring alignment and consistency across a large reader pool to deliver data that demonstrated the compound’s efficacy.
The study was a success and based on the imaging data delivered by Calyx Medical Imaging, the compound became the first FDA-approved immunotherapy for the adjuvant treatment of this RCC patient population.