Identify Unblinding Risks
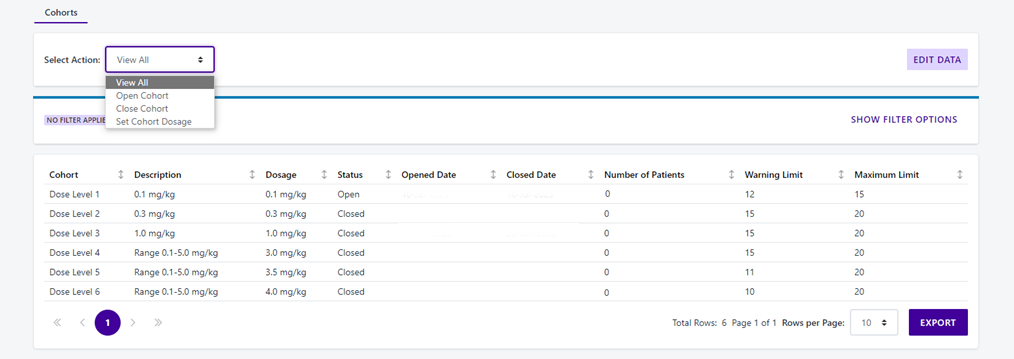
Every clinical trial runs the risk of unintentional unblinding.With 30 years of experience designing reliable RTSM solutions, Calyx IRT is the solution you can rely on to identify and minimize these risks.
Learn how Calyx’s processes drive the integrity of your trial by keeping unblinding risks to the minimum through