Study Management
At Calyx, our focus is ensuring that your recruitment goes to plan, even when mid-study challenges arise.

At Calyx, our focus is ensuring that your recruitment goes to plan, even when mid-study challenges arise.
Our experienced team of statistical design and trial supplies consultants work to understand and analyse your unique protocol requirements and develop a strategy to meet them initially while allowing for future flexibility if and when your study needs change.
Using our extensive experience from working with a range of protocol designs over many decades and in all therapeutic areas, we aim to identify risk and anticipate future needs so that we can recommend an approach that is both adaptable and future-proof.

Calyx IRT enables sponsors to easily make changes to the IRT design as and when needed. Through standard functionality, immediate changes can be made to meet common challenges faced during the course of a trial, from something as simple as an investigator correcting a patient’s DOB, to increasing enrolment caps to reflect changes in recruitment.
If your protocol allows for changes to study design, the IRT system can be designed to change immediately, for example adding new doses for new patient cohorts, or closing a treatment arm following a safety review.
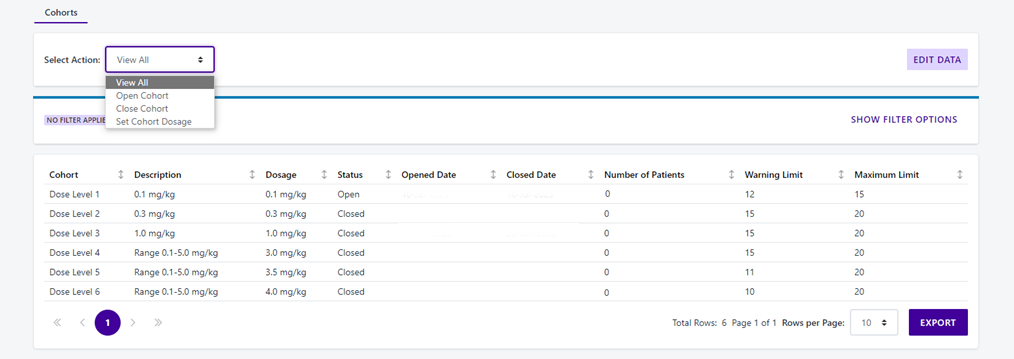
Calyx has built hundreds of cohort studies, including adaptive designs where the dose for successive cohorts is determined by the analysis of previous cohorts.
Calyx IRT minimizes data errors to improve clinical trial data quality by:
Calyx IRT further ensures data quality through integrations with other key eClinical systems, such as EDC, ePRO, and central labs, reducing the study team’s data reconciliation efforts and resulting in faster time to data analysis. And Calyx IRT is configured to reduce the risk of accidental unblinding, randomization imbalance, or mis-dispensing, resulting in increased protocol compliance and high data quality.
Additionally, through Calyx Patient Manager, investigative sites can make changes to patient data with immediate effect, based on permitted changes that are agreed upon by the sponsor during protocol design, which contributes to higher quality data.
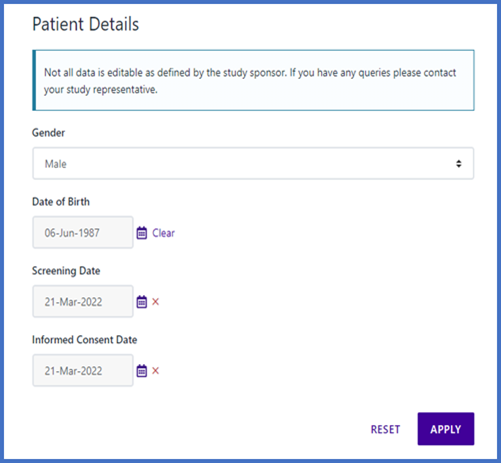
By integrating Calyx IRT with other eClinical systems, sponsors reduce the need for data duplication and reconciliation at the end of the trial. Calyx enables everything from simple IRT-to-EDC integrations to more complex, blinded central lab integrations and electronic patient reported outcome (ePRO) scoring.
To: Calyx
From: Top 5 Pharmaceutical Company
Subject: Calyx Medical Imaging helps get patients treated quickly
A very big thank you to Calyx Medical Imaging who worked outside of their turnaround timelines to review the scans that ultimately allowed the central team to approve enrollment to facilitate treatment of this patient as quickly as possible. This is a true testament of our values in ensuring that we get treatment as quickly as possible to patients that are in need.

To: Calyx
From: Top 5 Pharmaceutical Company
Subject: IRT team are great collaborators
I’m very happy with the collaboration we have seen with Calyx’s IRT team, their open communication, and their ability to discuss and solve issues.

To: Calyx
From: Top 5 Pharmaceutical Company
Subject: Calyx Medical Imaging Made this Approval Possible
A big thank you to the Calyx Medical Imaging team. This (approval) is also your accomplishment, and you should be very proud of the work you have done to make this possible. More approvals to come!
– Global Trial Manager
Liver Cancer Development Program

TO: Calyx
FROM: Veralox
SUBJECT: Calyx Medical Imaging – Thank you for meeting tight timelines
I am very impressed with the Calyx Medical Imaging team who have been incredible collaborators in helping us to advance our lead program. We especially appreciate the team working on tight timelines to help us meet our study start initiation goals. Given that we are a small company, we rely on our collaborators, and the team at Calyx works seamlessly beside us to help us advance our development programs. I look forward to continuing our partnership!
– Alicia Herr, PMP,
Veralox Therapeutics

To: Calyx
From: Top 5 Pharmaceutical Company
Subject: Calyx Medical Imaging – Transparent, Constant Collaboration
We worked very closely with Calyx on the pivotal uterine fibroid studies. Critical components of success included transparent constant collaboration with real-time communication at all levels between our study team and Calyx. This kind of communication throughout the life of the study helped to ensure our team had the information needed to make timely informed decisions and to deliver quality data for our deliverables. We were able to establish lessons learned from prior studies and implement those on this study. I’m looking forward to continuing our work together as we strive to bring more treatment options to women.

To: Calyx
From: Arrivent
Subject: Global Oncology Program – Calyx Medical Imaging Head and Shoulders above the Rest
I am writing to give testimony to the expert independent central imaging review services provided by Calyx in support of two of ArriVent’s global clinical oncology studies. Calyx has deep technical expertise in various tumor response criteria, imaging modalities, image collection logistics, data management and regulatory requirements. They gave ArriVent invaluable advice during protocol and charter development. This is coupled with a team of world-class highly specialized independent radiologists, imaging scientists, project managers, medical writers, a very efficient budget and contract group as well as superb internal processes and systems all of which contributed to a very smooth program kick-off for our lead molecule.
We have interviewed a number of different imaging CROs and Calyx is definitely head and shoulder above the others.
– Morgan Lam
SVP, Development Operations & Business Management,
Arrivent Biopharma

To: Calyx
From: Top 3 Pharmaceutical Company
Subject: Thank you Calyx Medical Imaging – Osteoporosis Trial Success
I would like to thank the Calyx Team very much for your contribution to this wonderful study outcome. Without you this marketing authorization would have never been possible.
-Medical Lead, Osteoporotic Trial,
Top 3 Pharmaceutical Company

To: Calyx
From: ProTrials
Subject: Pleased to partner with Calyx
We’re pleased to partner with Calyx and are confident that our customers will benefit from the scientific, medical, and clinical expertise they have honed during their 30 years of delivering reliable eClinical solutions to the clinical development industry.
– Christy Meyer
Director, Quality Insurance,

To: Calyx
From: LG Chem
Subject: Calyx IRT – Simplifying Complicated Randomization
I sincerely appreciate your cooperation and Calyx’s team effort for our program.
We could not have successfully completed the IRT development for both Ph3 studies without Calyx’s team expertise.
As you know, our Ph3 studies are very complex and your high degree of experience, knowledge and system (platform) were required in order to develop the IRT we wanted. Also, there were some challenges and concerns due to protocol amendment by LGC during IRT development.
But, we relied heavily on Calyx’s expertise in IRT to deliver these two complex trials in our Gout program. Calyx made recommendations on how to overcome some of the challenged we were facing, and it resulted in a solution that perfectly meets our needs.
I have been working for 13 years in clinical development area in pharmaceutical industry. Even if my experience was relatively short, I can tell that Calyx’s platform is very valid and elaborate compared to other IRT systems.
Thank you so much.
– Younghwan Jang,
Study Lead,
LG Chem, Ltd.

To: Calyx
From: ClinChoice
Subject: Partnering with Calyx for reliable data outcomes
We chose to partner with Calyx due to their tenured scientific, medical, and technical teams who possess a depth and diversity of experience in providing reliable data outcomes.
– Tiepu Liu, President,
Global Biometrics,
ClinChoice

test

