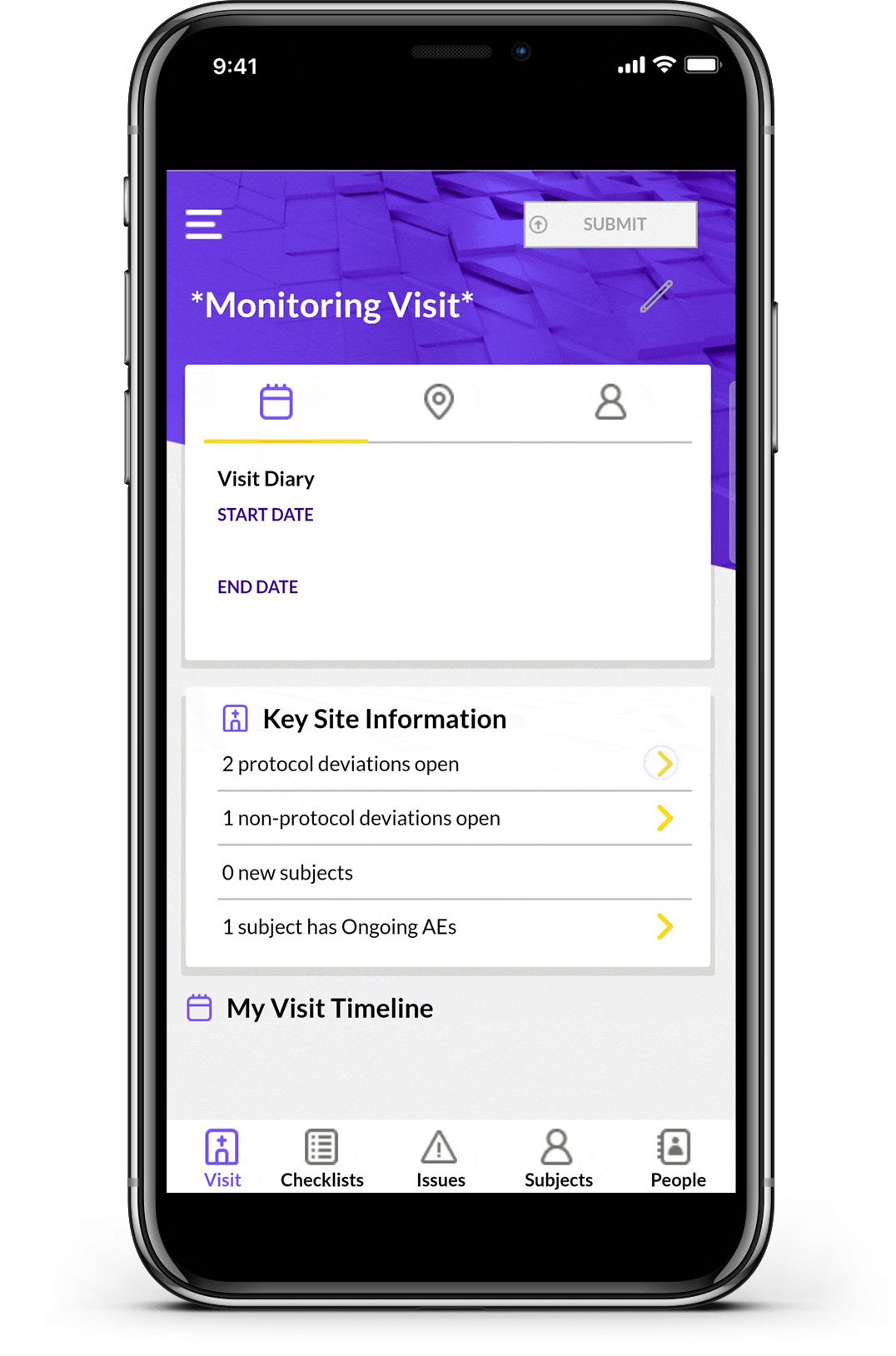
Calyx CTMS combines trial technology innovation, drug development expertise, and business process optimization to simplify the oversight of clinical trial operations and enable proactive risk management. The result? Timely execution from start to finish and superior data quality that informs decision-making to drive trial success.
Optimized through the delivery of 60,000+ trials, Calyx CTMS streamlines the entire operational workflow for end-to-end trial management, supporting clinical research programs across all phases, therapy areas, and design complexities.