During anti-tumor treatment development, imaging modalities, criteria, and regulators’ expectations change frequently. Without the direction of imaging scientists who work day in and day out in clinical trial imaging, it would be difficult, if not impossible to keep track of and react to changes during these critical and oftentimes, lengthy trials.
Successful medical imaging in solid tumor trials requires professionals with therapeutic experience, expertise in the modalities required to demonstrate safety and efficacy, and first-hand insight into what global regulators will look for in your submissions.
Here we present examples of scientific advances and regulatory changes that are currently impacting anti-tumor treatment research, demonstrating the need for an imaging partner who is immersed in the regulations, scientific learnings, and trends that could impact the success of your development program.
Example 1: Prostate Cancer
In prostate cancer research, PSMA-PET tracers are now commonly accepted for the detection of metastatic disease that was previously not visible on conventional imaging with CT/MRI and 99mTC Bone Scan. Patients and investigators are reluctant to perform conventional imaging with CT or MRI plus 99mTc bone scan when the more reliable and sensitive PSMA exams reveal more information about treatment efficacy or lack thereof.
Sponsors currently developing prostate cancer treatments are facing severe challenges in study design and meeting their protocol-defined endpoints based on conventional imaging alone. Even worse, as these pivotal trials take several years to complete, the modality and method of CT/MRI plus 99mTc Bone Scan may be outdated and no longer acceptable by the time the study closes.
In addition, there are several PSMA tracers available, they are similar yet not equal in their sensitivity and have slightly different uptake specifics. Plus, not all of these tracers are ready to be used in trials nor approved or available across different continents and countries.
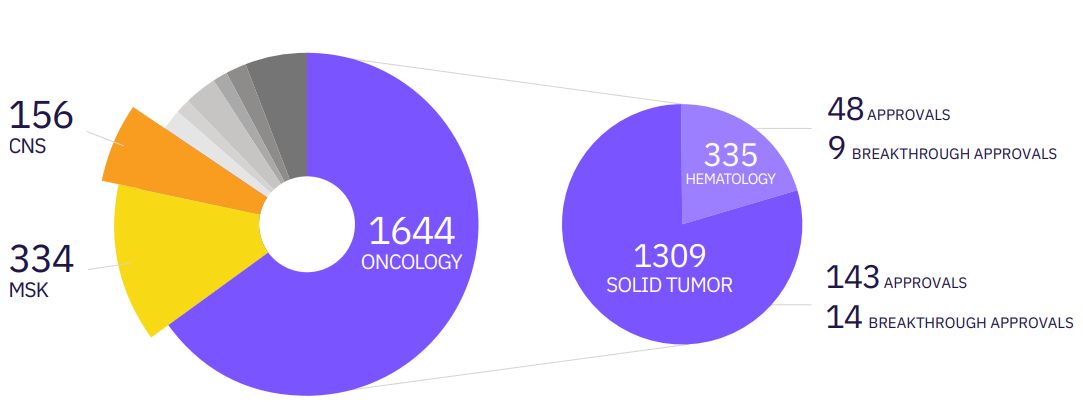
Calyx Medical Imaging has supported the approval of over 140 solid tumor treatment approvals, including this example of a phase III prostate cancer trial which led to the approval of a new treatment option for patients with mCRPC.
Example 2: Immuno-oncology research
Another example is related to the advances we’re seeing in immuno-oncology research. Recent findings are driving modifications to established imaging criteria, as evidenced in a Journal for ImmunoTherapy of Cancer paper (2022), marking the first time immune-related criteria show a correlation with overall survival as the most meaningful endpoint in the treatment of cancer patients.
A recent paper demonstrates irRECIST may provide more clinically relevant information and better treatment decision-making options than RECIST 1.1 alone in solid tumor image assessment.
Because immune-targeted treatment can initially cause a tumor to look as if it is progressing when it is not, clinical trial sponsors using RECIST 1.1 to assess treatment response may end up discontinuing patients who would otherwise remain in the study. Based on data from 1765 patients with 12 types of advanced solid tumors treated with avelumab monotherapy, the study found that irRECIST may provide more clinically relevant information and better treatment decision-making options than RECIST 1.1 alone in solid tumor image assessment.
As a result, Dr. Oliver Bohnsack (see sidebar) suggests that irRECIST can and should replace outdated RECIST 1.1 on all solid tumor trials going forward.

Calyx’s Dr. Oliver Bohnsack leverages his knowledge from having co-authored the immune-related response criteria (irRC, 2009), served as first author of irRECIST (2014), and co-authored Comparison of Assessments using RECIST and irRECIST by Manitz J. et al. (2020) as he designs and implements optimal imaging strategies for Calyx’s customers developing new oncology treatments
Solid Tumor Trials: Maintain More Patients with irRECIST
















