Leading up to our Virtual Panel on choosing the right biomarker imaging strategy in Phase I oncology trials, here we present a practical consideration of the tensions that exist in the use of imaging biomarkers and how to select the appropriate imaging modality to achieve objectives at different stages of the clinical development lifecycle.
Imaging Biomarker Tensions
Imaging biomarkers can provide a great deal of valuable information during the development of new medical treatments. But the role they play – and how to go about deciding which biomarker to use for your clinical development phase – will vary based on numerous factors.
As with many aspects of clinical development, there are tensions that exist as we strive to balance the use of medical imaging biomarkers. As discussed in our last blog, one of the primary tensions we encounter is the goal of using imaging biomarkers as a proxy for a change, such as a change in patient health, and how this may be affected by a reader’s bias.

“When considering the application of imaging biomarkers in a specific phase of study, we’re trying to balance different tensions and biases to achieve optimal results.”
– Luke Shipman, Director, Product Management, Calyx
Other tensions include factors such as invasiveness (the impact on the procedure to the patient), which can be measured in terms of the frequency as well as duration of image acquisition. For example, a very high-resolution CT scan is going to take longer than a more standard CT scan. We’re balancing access to a higher volume of patients versus the sensitivity of the scan itself. The more invasive the scan, the more sensitive the imaging output. The less invasive, the higher volume we have in terms of access to patients.
The same is true with imaging requirements related to access to sites and quality. Sophisticated imaging requirements will create high-quality imaging but may limit the volume of sites that can participate in trials.
As it relates to global phase III clinical trials, we tend to shift our balance in favor of access to global sites. And as a result, sometimes we make changes to imaging requirements to balance for a volume of sites that have those capabilities rather than very exploratory, specific imaging requirements. This is true across multiple dimensions; cost, criteria, complexity, modality, all of these are being balanced for when we’re selecting specific imaging biomarkers (Figure 1).
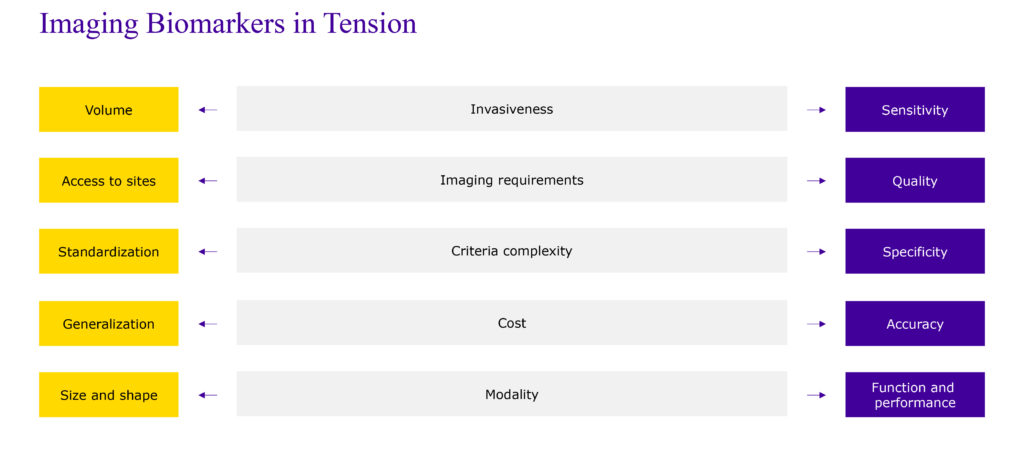
figure 1
Answering the Right Questions: A Prostate Cancer Example
The most important consideration when balancing imaging biomarker tension is, “What question do we want to answer?” Which we review in the context of prostate cancer below.
A prostate cancer trial may likely start with an assessment of target engagement. An imaging biomarker could help answer the question, “How well does the molecule reach the intended destination?” Since this is a functional assessment, we would consider using an FDG PET, which implies the use of a radiotracer to assess uptake at destination.
Advancing through the clinical trial phase spectrum, we might next use an imaging biomarker to examine the duration of response to surgical intervention. Do we see distant lesions or metastases? A metabolic biomarker, specifically PSMA will provide a high sensitivity to biochemically recurrent prostate cancer post intervention.
Moving forward, we start to explore therapeutic efficacy, i.e., “What’s the disease’s response to the intervention?” This requires consideration of the lesion’s physical characteristics ‒ the actual size and shape ‒ which requires an added radiology component to the biomarker repertoire. Typically, this will be CT and MRI.
As an aside, this phase of research presents a good example of the biases and tensions we tend to encounter with imaging biomarkers. We’ve all seen the increase of immuno-oncology therapies in clinical trials based on their promise of increased therapeutic efficacy. However, the nature of these therapies manifestly impacts how we consider what response looks like. A lesion that has an immuno-oncology response cannot be measured strictly by a reduction in size. In many cases, there will be a response in which that lesion actually increases in size at first. And so, we accommodate for that in terms of the way that we measure what response looks like and what imaging biomarkers to use.
In our prostate cancer example, the type of response we’re looking for could be either shrinkage, tumor necrosis, or flare response. So, in this case, we’d look at size and shape, but compare that against a functional response by using FDG pet.
Lastly, we might question the molecule’s safety, in which case we’d use a left ventricular ejection fraction assessment, which is performed via an echo or a MUGA.
Conclusion
Every day, we see new and exciting modalities and biomarkers. The appropriate selection of imaging modality and biomarker is directly linked to the success of a clinical trial. Successful studies employ effective biomarker selection strategies. These strategies achieve reduced timelines and higher study success rates by balancing the tensions that inherently exist in clinical trial data acquisition.
















