Overview
Calyx Medical Imaging is a leading imaging core lab with experience and proven capabilities in neuroimaging for clinical trials. Calyx Medical Imaging’s Central Nervous System (CNS) group understands the unique imaging needs of Alzheimer’s Disease (AD) trials and has the expertise and flexibility needed to effectively manage and scale these important clinical development programs covering early to late phases.
Calyx’s experience in AD includes confirmation of inclusion criteria (eligibility) and brain safety assessments throughout the study with rapid turn-around-times as well as advanced quantitative analyses for Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) data.
Case Study
Neuroimaging in Alzheimer’s Disease Trials
Modalities and Assessments
Calyx Medical Imaging is able to support several modalities and associated endpoints as required by the study protocol:
Positron Emission Tomography (PET) can be utilized to visualize and quantify specific neurochemical and molecular pathophysiology of the brain, by targeting brain glucose metabolism, amyloid accumulation, tau protein deposition or neuroinflammation. Standardized Uptake Value ratios (SUVr) or Centiloid scale approaches may be used as a quantitative assessment of amyloid and tau burden.
Magnetic Resonance Imaging (MRI) is commonly acquired to assess amyloid- related imaging abnormalities (ARIA-H and ARIA-E) by an experienced neuroradiologist. Such neuro-radiology reads are commonly performed for safety monitoring in AD clinical trials.
Furthermore, high resolution structural MRI is analyzed to quantify overall brain or regional structural changes, including cortical thickness, white and gray matter decomposition volumes, particularly in hippocampus and ventricles.
Diffusion Tensor Imaging (DTI) may also be used to pinpoint the white matter microstructural integrity and the associated changes for AD. The analysis can be conducted across the whole brain or region/tract- specific manner.
Functional Magnetic Resonance Imaging (fMRI) can be used to indirectly characterize brain activity and connectivity. fMRI can be implemented within a task-based or resting state paradigm depending on the targeted mechanism of action. For example, changes in Default Mode Network are commonly studied to understand the changes in brain connectivity during rest.
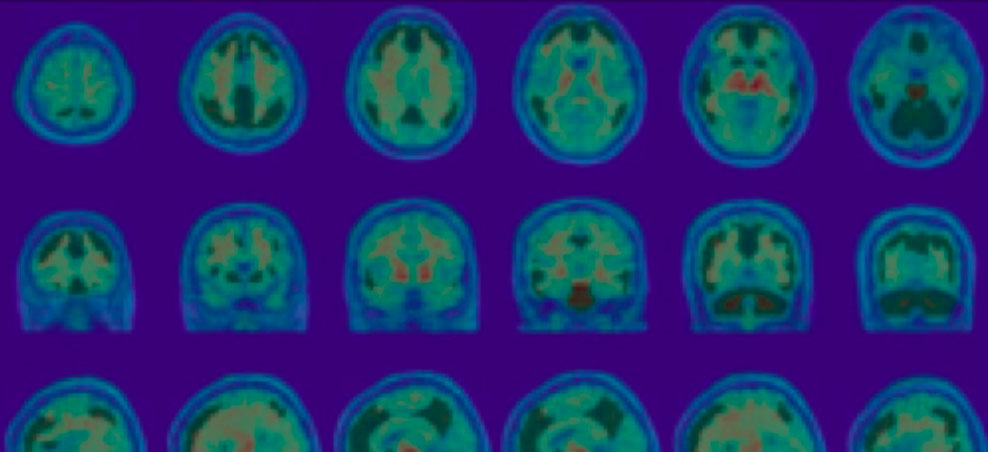
Gordon, E et al (2023). An automated pipeline for Centiliod quantification of amyloid-B using multiple “C-PIB-PET and F-PET tracers. AAIC
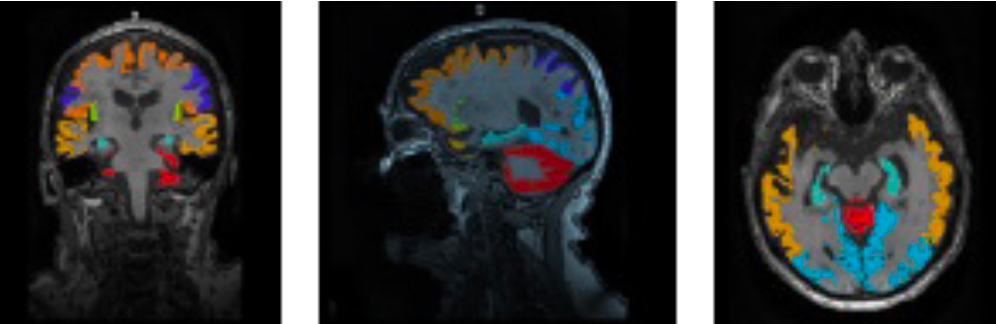
Enrica, C. et al (2022). Validation of an automatic tool for the rapid meastement of brain atrophy and white matter hyperintensity: QyScore®. European Radiology, 32(5), :2949-2961. https//doi.org/10.1007/s00330-021-08385-9
Partnering for Quantitative Analysis
Qynapse is a medical technology company with an AI-powered and proprietary neuroimaging software platform that creates the potential for earlier clinical precision for CNS diseases. Qynapse’s advanced analysis capabilities are now available through Calyx’s full suite of proven medical imaging services, enabling sponsors to de-risk and improve AD clinical trial outcomes.
Qynapse’s flagship solution, QyScore,® FDA-Cleared and CE marked, combines MRI scans and AI to produce rapid, actionable insights for objective brain scan analysis, enhancing diagnosis precision and drug efficacy assessment. Qynapse’s prognostic AI technology, QyPredict,® available for research use only, has the potential to predict disease trajectory and improve targeted patient selection in clinical trials.
Learn more about Calyx’s partnership with Qynapse.
Case Study
Calyx Medical Imaging supported an AD study in which qualitative assessments of MR imaging were required for enrolment. Central, independent readers assessed structural MRI scans to confirm there were no clinically relevant structural brain abnormalities, including hemorrhage (ARIA-H) and edema (ARIA-E). These safety assessments were conducted under expedited timelines to ensure the read results were shared with investigative sites in time to determine patient eligibility for the study.
For one participant, the independent neuro-radiologist reported two microhemorrhages in the frontal lobe, while the investigative site did not record any structural brain abnormalities. Upon discussion with the sponsor, the investigative site confirmed having missed the microhemorrhages and the central review results were taken into consideration accordingly for the patient’s eligibility determination.
Calyx Experience
Calyx Medical Imaging’s experience is drawn from managing over 2,600 trials to date which include more than 4.5 million images from roughly 145,000 sites globally. Within this experience is our management of 181 CNS protocols and over 22 Alzheimer’s Disease studies.
















