Medical imaging is often used in support of key study endpoints in prostate cancer trials, be it response-based or evaluating the time to recurrence or progress. It’s important to choose an imaging partner with a keen understanding of the challenges associated with these trials and who has the right experience to successfully deliver the critical imaging services needed to demonstrate treatment efficacy and help your development program succeed.
WHAT ARE THE CHALLENGES WITH IMAGING IN PROSTATE CANCER CLINICAL TRIALS?
Consistent and high-quality management of trials utilizing RECIST 1.1 with PCWG 2 or 3 criteria is not trivial. The recent addition of PSMA-PET imaging and its unprecedented accuracy adds further to the complexity of reliably addressing response to prostate cancer treatments.
HOW DOES CALYX DE-RISK IMAGING IN PROSTATE CANCER CLINICAL TRIALS?
With extensive regulatory and medical experience in designing and implementing imaging strategies in prostate cancer trials employing both PCWG and RECIST 1.1 criteria, Calyx can be relied on to help you obtain high-quality imaging data to meet your protocol objectives. Calyx’s in-house prostate cancer imaging experts are intimately familiar with the latest PCWG3 criteria and disease-specific imaging standards, including support for PSMA-PET analysis. Our team can help you navigate common challenges and implement solutions for these complex imaging criteria. Calyx is actively supporting three prostate cancer studies that required the supplemental add-on of PSMA-PET imaging into running trials. We are also in collaboration with a client using a CAR-T cell therapy approach to prostate cancer and are actively engaged with Professor Wolfgang Fendler, author of the international PSMA-PET guideline and of novel criteria needed to navigate PSMA-PET-based outcome analysis.
Download This Case Study
ADDITIONAL RESOURCES:
Assessing Prostate Cancer on Imaging in Clinical Trials
Watch Webinar
Calyx Medical Imaging
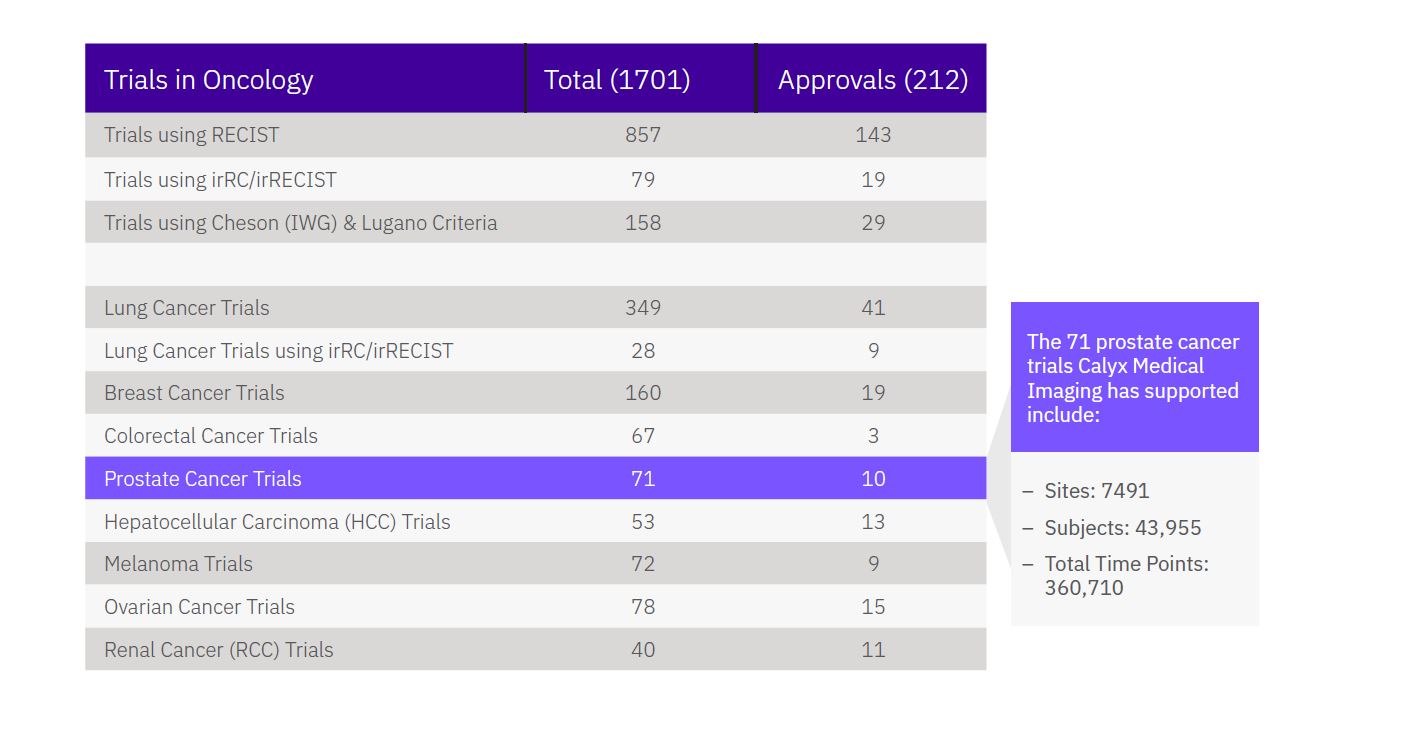
Prostate Cancer Imaging Expertise
SCIENTIFIC EXPERTISE
Calyx’s scientific and medical team of over 80 full-time imaging experts ensures that we design and deploy the right solutions tailored to meet your research requirements. Our oncology team is led by Dr. Oliver Bohnsack, who leverages his extensive knowledge of RECIST and irRECIST as he designs and implements optimal imaging strategies for Calyx’s customers developing new oncology treatments.
TRIAL EXPERIENCE
Calyx has extensive regulatory and medical experience in the design and implementation of imaging in prostate cancer clinical trials employing PCWG and RECIST 1.1 criteria. Calyx has managed the imaging component of numerous global Phase II and Phase III prostate cancer trials, including protocol development support and the delivery of effective tools to harmonize site/central discordance.
Oliver Bohnsack, MD, PhD, MBA, Vice President, Scientific and Medical Services, Head of Oncology, Calyx
Case Study
Top Five Pharma Seeking Approval for Hard-to-treat Prostate Cancer
One of the world’s top five pharmaceutical companies was in late-stage development of a new treatment for metastatic, castration-resistant prostate cancer (mCRPC). Because this advanced form of prostate cancerdoesn’t respond well to currently available treatments it is particularly challenging for patients and medical professionals alike.
The company selected Calyx Medical Imaging to support its pivotal Phase III trial, which enrolled over 900 patients from over 150 worldwide investigative sites. The imaging data collected throughout the three-year study supported the sponsor’s primary efficacy endpoint and was included in their submission for regulatory approval. For this study, Calyx designed and delivered robust Medical Imaging services that included:
- A comprehensive imaging charter, following the Prostate Cancer Working Group 3 (PCWG3) criteria, developed by Calyx’s professional medical writing team
- Proven, scalable services to drive the collection of CTs, MRIs, and bone scans, which involved:
- Training of investigative sites on the correct image capture procedures
- Collection and quality review of over 22,000 CTs, MRIs, and Bone Scans
- Blinded Independent Central Review (BICR) of imaging reads by 20 expert reviewers
- Rigorous quality control processes monitoring image quality and reviewer performance throughout the study duration
- A dedicated scientific/medical team with expertise across multiple oncology indications and imaging modalities
Calyx’s scientific and medical team (SciMed) remained intact over the four-year study, consulting with the sponsor and supporting the investigative sites and reviewers throughout to ensure the data derived from the important imaging analysis was of the highest quality.
Calyx met all the deliverable dates throughout the study and supplied reliable imaging data to support the study’s primary efficacy endpoint, demonstrating that the treatment significantly reduced the risk of early disease progression or death compared to traditional treatment approaches.
As a result of the robust and reliable data supplied by Calyx Medical Imaging, the new and better treatment was approved and is now giving patients a safe and more effective treatment option for mCRPC.