Authors: Oliver Bohnsack M.D. Ph.D. MBA, Manuela Lesch, RT,
Elif Sikoglu, Ph.D.
Editors: Elizabeth Dalton
Brain metastases from common solid tumors are frequently seen – they are present in up to 50% of patients with lung cancer, breast cancer and melanoma.
The optimal workflow for assessing brain metastases, in the context of solid tumor clinical trials, is still under discussion as the presentation and progression of brain metastases as well as their response to treatment may be unique.
Within this context the primary objective of clinical trials is to determine whether the systemic treatment shows an overall benefit on the patient outcome or is focused on CNS benefit only. In Calyx’s experience, regulatory authorities emphasize the overall patient outcome which integrates the CNS component, based on the notion that a systemic treatment affects the whole patient and not only one part of the body.
However, the treatment effect may differ between body and brain and, in the context of drug development programs, sponsors often need robust and specific information about the efficacy of a novel treatment including CNS metastases.
The aim of this paper is to address the recommendations by the Response Assessment in Neuro-Oncology (RANO) working group by providing guidance to biotech and pharmaceutical sponsors based on lessons learned from clinical trials with the implementation of RANO criteria for brain metastases and associated workflow.
Some of the major challenges addressed in this white paper:

Cognizant of these common challenges,
Calyx recommends the following:
- Standardize brain imaging
- Avoid sampling bias at follow-up timepoints
- Standardize assessment and managing reader performance
- Implement an independent read paradigm for efficacy
- Define the right patient population
- Follow efficacy criteria for brain metastases
- Follow efficacy criteria for body imaging
- Leverage the (neuro)oncologist
1. Standardize brain imaging
CT scanning is less sensitive than MRI for detection of brain metastases, thus brain MRI is strongly recommended in clinical trials when response determination in brain metastases is required.
It is Calyx’ recommendation that the minimum MRI requirements be consistent with respect to:
– Sequences: 3D T1w pre- and post-contrast, 2D T2 and/or 2D FLAIR and DWI
– Slice thickness with a maximum of 3mm (1.5mm preferred) for T1w pre- and post-contrast
– Consistent slice positioning between visits
– Assurance of contrast penetration into the brain, i.e. sufficient delay time prior to acquisition of post-contrast T1w sequence
2. Avoid sampling bias at follow-up timepoints
The RANO article states: “CNS lesions should initially be re-assessed by MRI at protocol-specified intervals 6–12 weeks apart, although there might be specific circumstances in which longer (or shorter) intervals are desirable. For patients who remain stable for extended periods of time, a longer interval between scans might be appropriate.”
Currently, many protocols require baseline brain imaging and then anticipate clinically triggered imaging of the brain. This approach results in reliance on unscheduled and irregular imaging and this can be a progression indicator. ODAC has consistently warned sponsors that inconsistent timing of scans using Progression Free Survival (PFS) endpoints in studies of metastatic tumors of the body can be challenged from a statistical perspective. Why should PFS endpoints in studies of metastatic tumors of the brain be exempt, especially as the MR imaging is not contributing to an increase in radiation exposure?
3. Standardize assessment and managing reader performance
Even though the RANO for brain metastases criteria is clearly defined in the literature, the application of assessment criteria may differ among study sites. Hence, central reads play an important role in standardization of the assessment and providing unbiased and blinded data for realizations of clinical trial endpoints. To this end, Calyx employs several strategies to enhance independent reviewer performance:
– Engage the smallest number of readers as possible
– Have neuroradiologists read MRI and/or CT studies of brain for CNS specific assessment criteria
– Have radiologists with subspecialty training in body radiology read the body imaging
– Provide consensus and rigorous training to the selected readers
– Provide easy references and rules to the readers
– Monitor reader performance to ensure adherence to the criteria and take corrective/preventative actions as needed
4. Implement an independent read paradigm for efficacy
Lack of prospective planning concerning CNS assessments in trials of systemic treatments will pose severe challenges on the collection of consistent data to support endpoints specific to the CNS. Additionally, criteria that have been developed for the body do not capture the criteria needs of the CNS. Calyx therefore recommends:
– Readings for response in the brain and response in the body should be performed separately with specified assessment criteria and the results captured in separate Case Report Forms (CRF). An oncologist, or better still a neurooncologist may integrate the two radiologists’ assessments with any clinical information available and provide an overall patient status in a third CRF.
Alternatively, the overall assessment may be programmatically derived utilizing body and CNS response assessments.
- If PFS or response of CNS metastases is the primary endpoint in a trial for regulatory submission, sponsors should consider double reading with adjudication of this CNS assessment. Otherwise, single read is acceptable.
- If PFS or response at the patient level is the primary endpoint in a trial for regulatory submission, double reading with adjudication of the body assessment is recommended.
– If eligibility review for trial inclusion of CNS imaging is required for enrollment or stratification, it is ideal to ensure reader consistency by assigning the same neuroradiologist for all follow up efficacy reads for that patient.
5. Define the right patient population
Calyx has managed a significant number of metastatic brain imaging studies. Protocols commonly and specifically require:
– No brain metastases at screening,
– No active brain metastases at screening,
– Measurable brain metastases at screening, or
– Brain metastases that are progressing at screening despite local treatment.
The stratification regarding brain metastasis is often done by investigator sites. However, we found in a recent study that approximately 20% of all screened patients thought to be clinically free of brain lesions actually had brain metastases verified by brain imaging. Hence, in recent studies, Calyx has been utilizing eligibility reads and informing the sites whether follow-up brain imaging is required.
Preparation is needed to minimize errors of enrollment regarding brain metastases, which includes training investigators and study coordinators on which type of protocol specific imaging exams are required and whether the scans will need to be sent for BICR (Blinded Independent Central Read). It is often challenging to collect and interpret imaging acquired prior to enrollment into a particular clinical trial. However, if determination of prior progression is an eligibility criterion, CNS imaging done prior to screening, such as images from radiation therapy planning or prior diagnostic exams, must be collected and reviewed. Clinical information, such as prior local treatment information, may also be required for eligibility. If so, a study specific “baseline clinical” form can be used to list all prior radiotherapy or local intervention details.
For example, if eligibility requires a measurable lesion and prior local treatments are allowed, Calyx recommends only lesions with a minimal longest diameter of 10 mm and meeting any of the following criteria to be considered measurable:
– New brain metastasis
– Brain metastases without any prior local treatment
– If local treatment for brain metastases occurred:
- Either an existing treated lesion must have progressed (20% increase in longest diameter)
- Or at least one measurable brain lesion must have remained free of local treatment

6. Follow efficacy criteria for brain metastases
Baseline Selection of Lesions
Target lesion selection and measurement
– Size: ≥ 10mm in long axis and short axis 5mm
– Contrast enhancing
– Clear and reproducible lesion boundaries
– Avoid selecting necrotic or cystic lesions as target lesions if other solid lesions are present
– Avoid selecting necrotic or cystic lesions as target lesions if other solid lesions are present
– Measure on axial plane, preferably post-contrast T1w
– Slice selection: the slice with the longest in-plane diameter should be chosen at baseline for each target lesion’s measurement
– Maximum of 5 brain parenchyma lesions
– Calculate the Sum of Diameters (SOD) which is defined as the sum of the longest axes of all target brain lesions
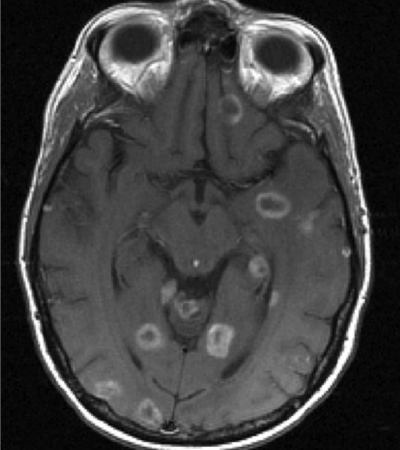
Target lesion selection and measurement
– Include all measurable lesions not chosen as target lesions
– Lesions < 10mm in long axis or < 5mm in short axis
– Can be contrast enhancing or non-enhancing
– Lesions with non-clear boundaries
– Multiple lesions in the brain may be grouped together and assessed collectively
– Skull and scalp lesions will be assessed by the body radiologist, not the neuroradiologist
– There is no limit on number of non-target lesions
Follow-up lesion assessment
Target lesion selection and measurement
– Measure all target lesions
– Continue to measure lesions even if they are < 10mm. If a lesion is visible but is too small to measure, a default value of 5 mm should be used
– Calculate the SOD
– Calculate the % increase from the nadir SOD (smallest SOD of any timepoint including baseline)
– Calculate the % decrease from the baseline SOD
– If a lesion separates to form discrete lesions on a subsequent imaging timepoint, the longest diameter of each lesion will be calculated and reported separately
– If initially separate target lesions merge (without a plane of separation), the longest diameter of the resulting lesion will be calculated and recorded for one of the original target lesions. 0 mm measurements will be entered for the other target lesion(s) and pertinent comments will be recorded.
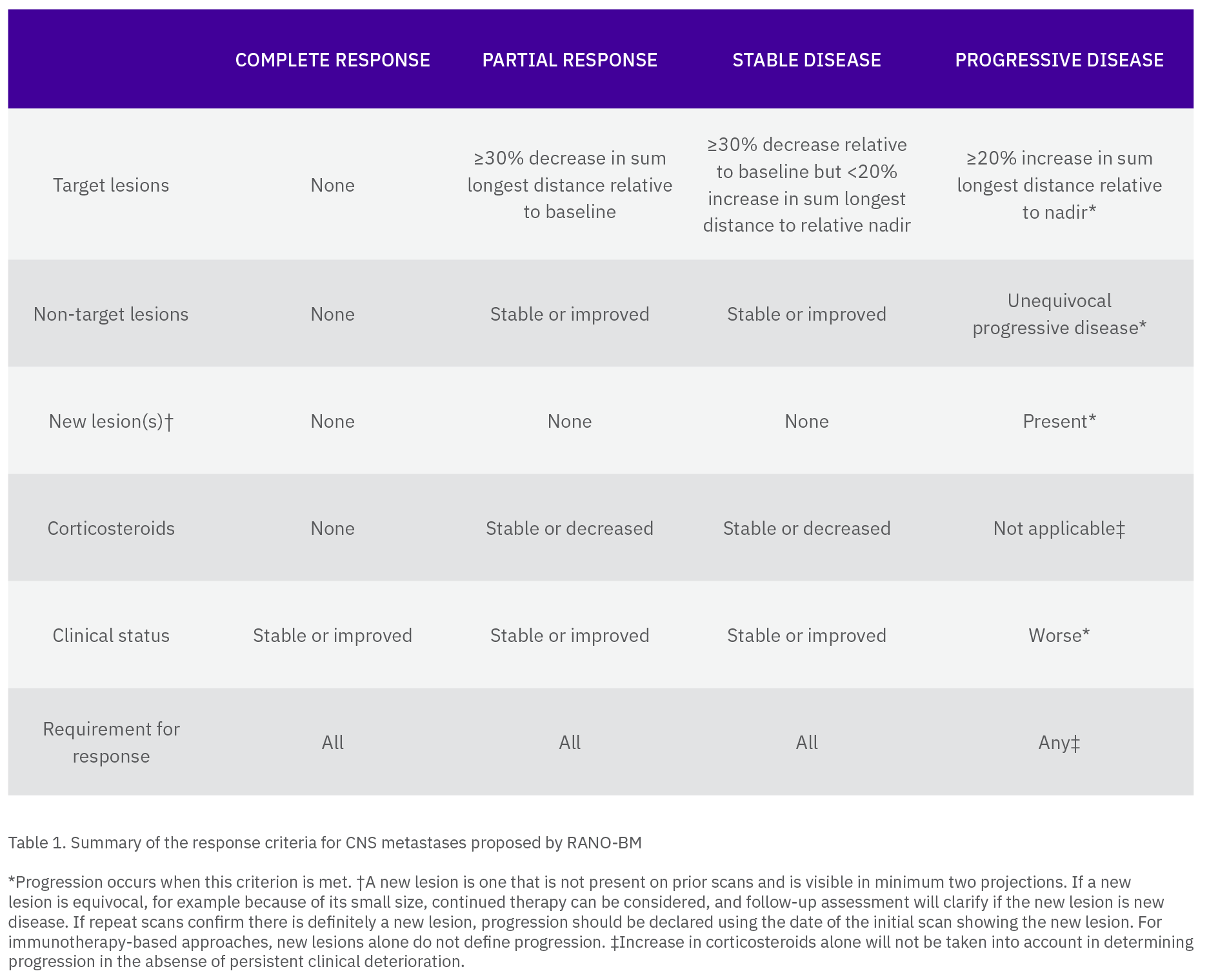
Determine the target lesion overall response according to the following criteria:
– CR (complete response): Absence of all lesions and the entire brain is evaluable. CR is also achieved when all target lesion(s) do not show any Gad enhancement but are completely necrotic
– PR (partial response): ≥ 30% decrease in the SOD from baseline
– SD (stable disease): Neither growth sufficient to qualify for PD nor response sufficient to qualify for PR
– PD (progressive disease): ≥ 20% increase in the SOD from nadir and at least a 5mm absolute increase in SOD from nadir, with or without worsening of neurological symptoms
– NE (not evaluable): Incomplete imaging or change in modality preventing precise measurement
Non-target lesion assessment (individual non-target lesion or group)
– CR: All lesions absent and the entire brain is evaluable. CR is also achieved when all non-target lesion(s) do not show any Gad enhancement but are completely necrotic
– Non-CR/Non-PD: Persistence of lesion(s)
– PD: Growth which is sufficient to determine unequivocal progression of non-target lesion(s) with or without worsening of neurological symptoms
– NE: Incomplete imaging or other causes preventing assessment of lesion(s)
Determine the non-target overall response according to the following criteria:
– CR: All lesions are absent, and all lesions are evaluable. CR is also achieved when all non-target lesion(s) do not show any Gad enhancement and are completely necrotic
– Non-CR/Non-PD: Meets none of criteria above (not PD, not NE, not CR)
– PD: Any individual non-target lesion (or group) response is PD
– NE: Any lesion is not evaluable, and no lesions are PD
New lesions
Equivocal
– A new finding which is present but may not represent malignancy or a new lesion which may be present but is either too small, too ill-defined to be certain, or is a potential artifact
– Equivocal new lesions do not trigger PD at the current timepoint. If an equivocal lesion becomes unequivocal at a later timepoint (e.g. due to growth), the time of progression is dated back to the timepoint of that lesion’s first recognition
– Equivocal new lesions prevent an overall assessment of CR
Unequivocal
– A lesion, which is considered new and malignant, that is not attributable to differences in scanning technique or findings, irrespective of its size
– Unequivocal new lesions trigger PD
Special circumstances
– New significant edema with a mass effect if it corresponds to a metastasis or leptomeningeal disease are considered new lesion and will trigger PD
– Lesions detected in areas not scanned and documented at baseline are considered unequivocal new lesions and will trigger PD
Reappearing lesions
– If overall response at prior timepoint is not CR:
- Re-measure the lesion and add the value to the SOD. PD will be triggered if the SOD increases >20%, the actual SOD increase is ≥ 5 mm, and at least one other target lesion of any size is present
– If overall response at prior timepoint is CR:
- Re-appearance of any target lesion is considered an unequivocal new lesion and will trigger PD
– Any unequivocal reappearing non-target lesion will trigger PD
– Hemorrhage is not a non-target and is not a new lesion
Overall Timepoint Assessment
The Overall Timepoint Assessment will be derived from the Target Lesion, Non-target Lesion and new lesion assessment. Clinical data may be included into the overall assessment as outlined in figure X by a neuro-radiologist or a neuro-oncologist.
7. Follow efficacy criteria for body imaging
With the focus on CNS metastases from solid tumors Calyx recommends that the assessment of body imaging is reported on a separate eCRF by separate readers, apart from the CNS imaging eCRF.
The read should be performed by a different radiologist than the neuroradiologist reading the CNS imaging, preferably a reader with advanced training in body imaging and experience with RECIST 1.1 or in the disease-specific applicable criteria. Imaging of the head and neck other than intracranial CNS will be included in the body imaging review.
8. Leverage the (neuro)oncologist
After the CNS and body images have been read, a (neuro)oncologist may:
– Evaluate the CRF completed by the neuroradiologist during the CNS review
– Evaluate the CRF completed by the body radiologist during the body imaging review
– Review clinical data
– Evaluate treatment related signs and symptoms
– Evaluate tumor related signs and symptoms
– Complete a separate CRF
Conclusion
As with any other criterion involving tumor measurements, the key to accurate, reproducible assessment of response to treatment in both clinical practice and clinical trials is the involvement of radiologists, in this case neuroradiologists, experienced in oncological imaging. The assessment of response not only requires precise tumor size measurements, but also requires an in-depth understanding of the nuances and complications regarding presentation of disease within the brain as well as potential cancer therapies.
Calyx‘s aim is to reduce variability for trial sponsors by working closely with the sites to acquire the best possible images to undergo central read processes where the readers are diligently trained with predefined analysis rules.
References
1 . Response assessment criteria for brain metastases: proposal from the RANO group,Lancet Oncol. 2015 Jun;16(6):e270-8. doi: 10.1016/S1470-2045(15)70057-4. June2015
















