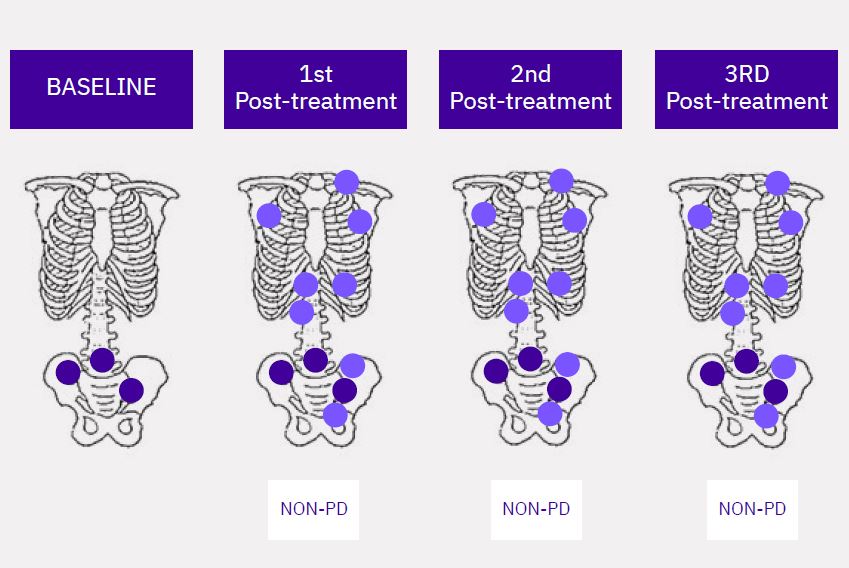
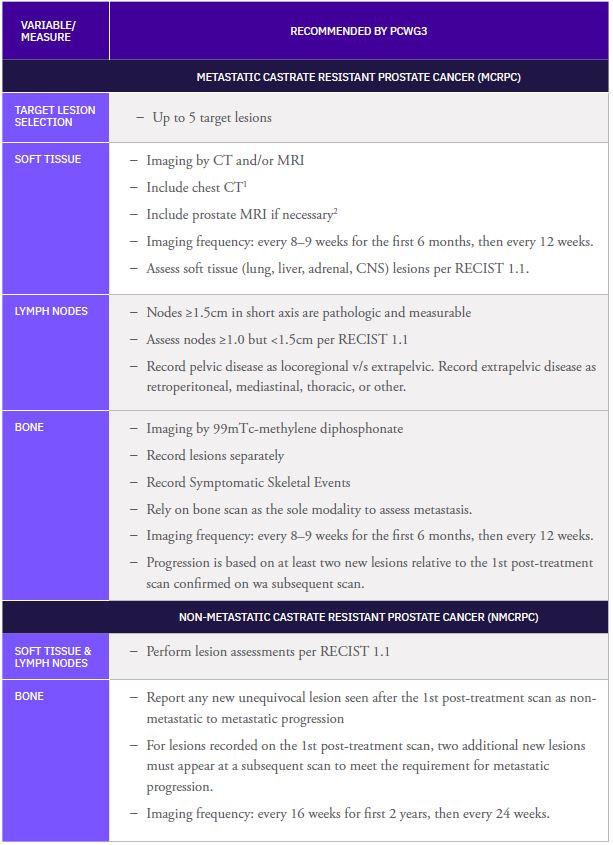
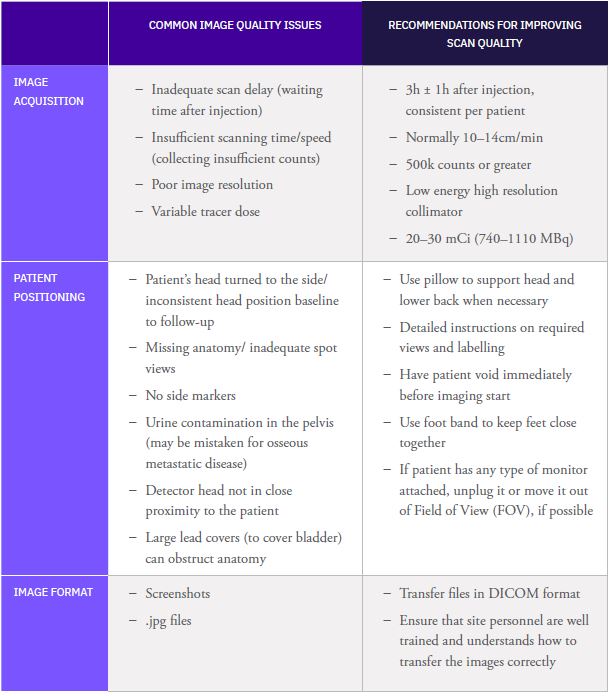
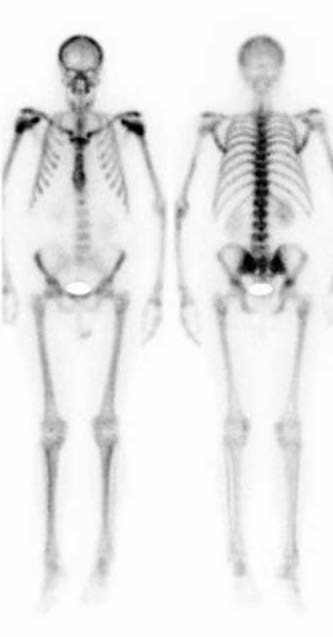
Calyx Medical Imaging Delivers More Precise Brain Tumor Assessments
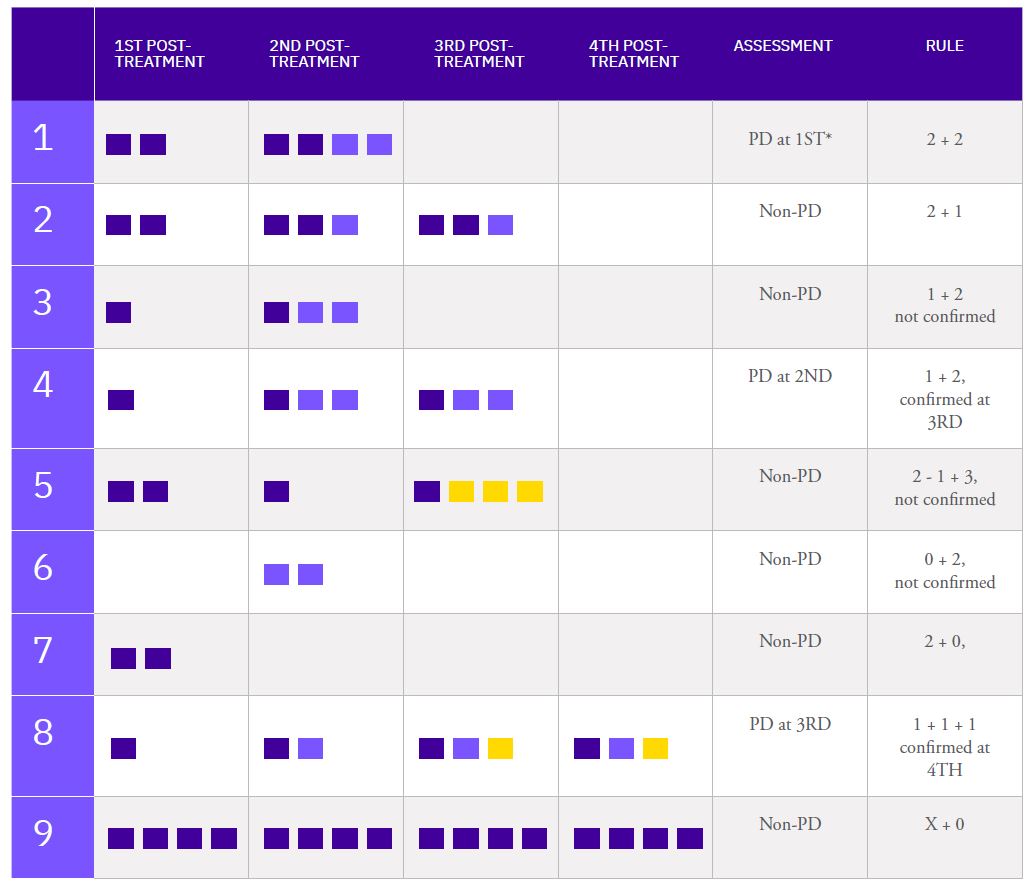
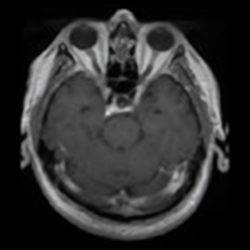
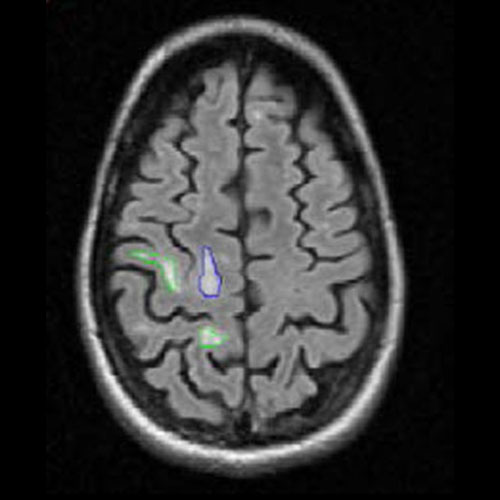
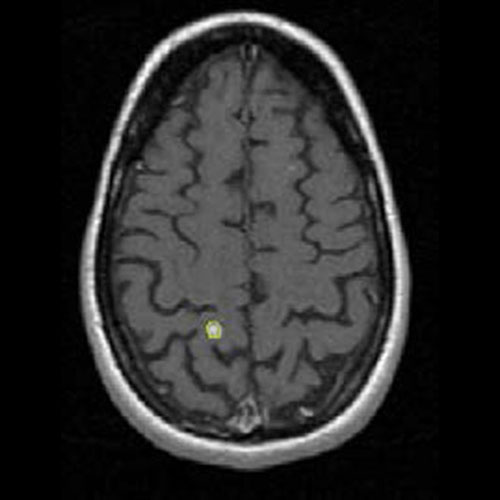
Assessing brain tumors in a standardized manner to characterize treatment response is complex. Although RANO criteria are commonly utilized for neuro-oncology clinical trials, there is a growing need for objective and quantitative markers to assess tumor size and characteristics. However, volumetric tumor assessments, which currently need to be conducted manually by neuroradiologists, are time-consuming and challenging.
To overcome this, Calyx Medical Imaging partners with Neosoma, an innovative medical technology company whose AI-based neuro-oncology software device is shown to achieve high accuracy in tumor volume measurement and consistent, faster, and more precise assessments of tumor change.