Creating and developing content for a program of clinical trials involves a balancing act between leveraging efficiencies, maintaining program-level consistency, and ensuring scientific integrity. One approach to managing such content is to utilize program-specific document templates, but templates leave the door open to undesirable edits that do not serve to increase efficiency, consistency, or scientific integrity.
In fact, stylistic edits and changes that do not serve to change the actual meaning of the template content create confusion when comparing documentation across the program in the hopes of understanding the key differences and associated rationale. Imagine, for example, a program of five studies in which the table of contents for a given document type was aligned for four out of five of the studies, but for the fifth study, a key stakeholder decided that a given section should be moved to another location in the document or nested below a different heading as compared to the other four documents. Assuming the audience of the fifth document was familiar with the previous four studies, the reader of the fifth document would be perplexed as to why an entire section was removed from the fifth document and would waste time searching for the missing section and/or trying to understand why that section did not apply to that final study.
More seriously, changes to the study approach based simply on personal preference rather than on scientific justification can lead to a situation where data across studies in the program are not easily comparable (i.e., apples to oranges rather than apples to apples). In both scenarios (i.e., stylistic and content edits), a consistent medical writer working across the program of studies may serve as the gatekeeper and liaise between trial teams to align language and the approach as closely as possible, but this decreases efficiency and cannot completely prevent inevitable unnecessary differences in the content.
Following recent FDA guidance that defined the imaging charter document as “either a single document or a series of technical documents,” it became possible to reorganize content into two separate documents. Using the imaging charter as an example, evaluation of a given program-level template revealed that 80% of the content was expected to remain consistent across studies in the program and that only up to 20% of the content could reasonably be expected to vary due to individual study needs and differences (Figure 1).
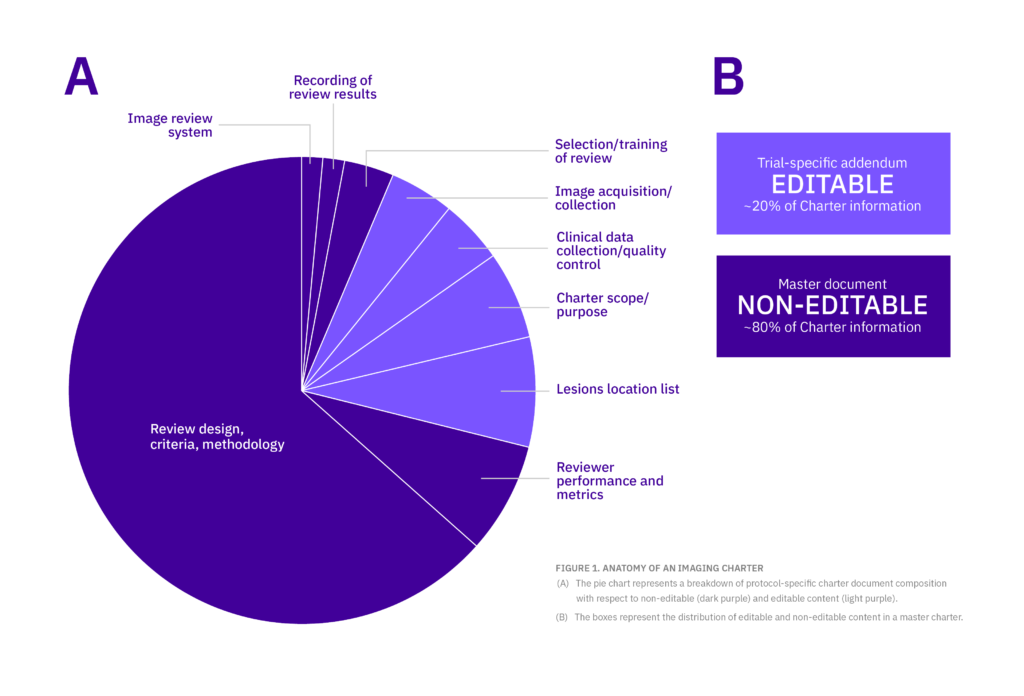
Following this evaluation of the imaging charter template, the content could be reorganized into two documents. One root-level document, the master document, contains the locked content that should not change across a program of studies; a second document includes only the protocol-specific clarifications and rationale for any changes from the program-level approach, if applicable.
Application of this approach resulted in not only improved efficiency but also in increased consistency and scientific integrity, by discouraging any stylistic and unjustified trial-level alterations and teasing out the trial-specific information, thereby increasing transparency. Another added benefit was in the case of a required and justified program-level change. Such a change could be applied once to the master document, thereby eliminating the need to make the same edit to each individual study document and also proactively removing the potential for additional edits to be made during document revision, which could potentially result in additional increased variance across the program.
Splitting information across a locked root level document and a second document that can be adapted for trial-specific information may not apply to every type of content. However, a modified approach that uses this same concept can be applied. For example, program-level templates can be utilized but can be modified to include locked sections of content.
What is important to consider before applying a master document approach for a given trial document is whether the bulk of the content is specific to a given process, system, or program, and is not expected to change significantly at the program level. Once this is determined, the content can be organized in a new way to support improved efficiency, consistency, scientific integrity, and transparency.