Craig Mooney, VP, Scientific e-Tech Enabled Services, Calyx
In this dynamic industry with so many moving parts, some consider the use of IRT for randomization and trial supply management (RTSM) to be an easy-to-master technology, even touting that becoming an expert can happen in just one day.
This couldn’t be further from the truth. Minimizing the strategic planning that must go into the design of an effective IRT system, as well as the proper management of its lifecycle demonstrates a lack of understanding about how impactful IRT can be to clinical trial outcomes. There are significant consequences that can arise if a study’s IRT system isn’t implemented with insight, expertise, and precise focus on the protocol’s needs.
Randomization Risks
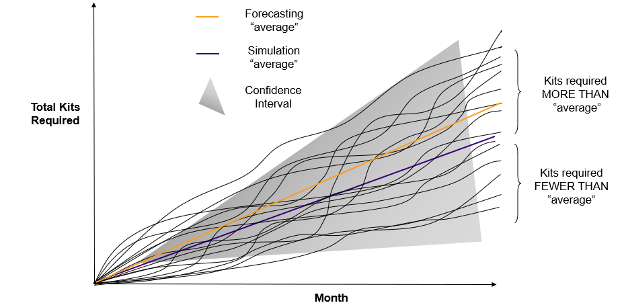
For example, if randomization is implemented incorrectly, the scientific integrity of the entire study could be called into question. This has been evidenced across numerous studies where a sponsor’s primary objectives or endpoints could not be met due to a failed randomization implementation.
Effective and reliable randomization requires more than loading a list. It involves determining how the list will be implemented, i.e.:
- Should blocks be assigned by a site when the site is activated?
- Should they be assigned dynamically to a site as needed?
- How will mis-randomizations or ‘randomized in error’ be handled?