Proven. Adaptable. Trusted.
There’s nothing more important than keeping clinical trial patients safe. Which means ensuring they receive the right medication on time, every time.
That’s why, since 1993, the world’s leading biopharmaceutical companies and CROs have repeatedly turned to Calyx to meet their varied randomization and trial supply management RTSM) needs.
With Calyx IRT, you gain confidence in RTSM through an extremely robust system, built on decades of experience, and delivered by the most experienced project teams in the industry.
KEY FEATURES
- Advanced randomization management, suitable from simple blocked and stratified randomization designs to more complex adaptive trial designs
- Flexible and robust medication management algorithms, including automated workflows and self-service tools
- Intuitive site functionalities configured to match protocol requirements and increase protocol compliance
- Reporting adapted to the needs of each user type
KEY BENEFITS
- Reduce the risk of unblinding, randomization imbalance, or mis-dispensing by working with the most experienced teams in IRT
- Meet key milestones – Calyx teams consistently deliver excellent quality on time
- Reduce effort during IRT system setup thanks to the expertise of Calyx staff who make your decision-making process simpler
- Leverage the highly integrated IRT solution to increase data quality, reduce effort in data reconciliation, and reduce the risk of unblinding
- Decrease vendor oversight by leveraging Calyx’s excellence in project management
- Increase your confidence in supply chain settings by engaging with Calyx supply chain experts
- Reduce the cost of drug management through advanced IRT settings
Optimized through the delivery of 4,500+ trials to date, Calyx IRT is an RTSM solution you can trust. Every time.
Reduce trial risks, ensure compliance
With Calyx’s robust functionalities and proven processes, you gain confidence in your ability to control the risks of:
- Unblinding
- Imbalanced randomization
- Mis-dosing or mis-dispensing
- Supply chain disruption
Calyx IRT allows you to focus on trial execution without worrying about impacting the validity of your trial or the safety of your patients.
HIGHLIGHTS
- Central randomization list, generated by a dedicated team with experience over thousands of trials
- Statistical designers with average 7+ years’ experience in IRT and 250+ adaptive trial designs delivered
- Secure data blinding, resulting in safe navigation through Calyx IRT and safe communication with our helpdesk
- Risk of unblinding reduced to 0.0001% per transaction
- Medication management controls automatically restock sites and alert you of depot low stock, resulting in medication on site whenever patients need it
- Expiry controls that remove the risk of medication expiring in the patient’s hands
- Drug accountability, return, and destruction workflows allow you to track medication throughout its lifetime, and easily account for each single kit
Known for its ability to adapt to any clinical trial need, regardless of complexity, Calyx IRT is an advanced RTSM solution that takes the worry out of randomization and supply management so you can concentrate on study success.
Boost your productivity
With Calyx’s proven processes, you spend less time on IRT considerations and more time on trial execution.
From initiating the IRT design to database lock, Calyx’s expert project teams will recommend the best functionalities to meet your protocol requirements, selecting from our set of standards. Your time in vendor management will be reduced thanks to Calyx’s IRT expertise and broad experience managing unplanned situations. You will have access to tools that help you adapt to actual site recruitment.
HIGHLIGHTS
- Existing set of standard functionalities to select and adapt to your own standards, to further reduce IRT setup time
- As little as 4 weeks from requirements approval to UAT
- Integrations with eClinical suites (EDC, ePRO, central labs, supply management, etc.); Calyx IRT successfully exchanges more than 10,000 files with other systems daily
- User acceptance testing script writing and execution service simplifies IRT setup
- IRT-dedicated helpdesk, specialized in managing the challenges of patient and inventory management for your sites and trial teams
- Calyx supported 750+ live studies during the COVID pandemic, keeping all patients supplied with medication
- IRT self-service tools allow you to quickly adapt to varying site recruitment levels, change/add supply strategies, and activate new countries and depots
Reduce your cost of IRT ownership with Calyx IRT.
Reduce drug management costs
Calyx IRT is more than just randomization and clinical supply management solution, it also enables you to reduce the cost of drug management. Calyx project teams are experts in optimizing clinical supply chains through IRT settings. For each protocol, they assess what is most prominent between the cost of shipping to sites and the cost of drug, and recommend how to apply IRT settings that will reduce the overall cost of drug management.
HIGHLIGHTS
- Advanced IRT settings for site supply management, reducing drug wastage beyond the typical buffer stock and predictive drug shipment, such as:
- Randomization code look-ahead
- Automated recruitment-based supply adaptation
- Fractional predictive shipments
- Statistical designers with average 7+ years’ experience in IRT help you to best balance the cost of drug and cost of shipments
- Supplies management adapting to central and local sourcing strategies
- Self-service tools provided to clinical supplies management team to adapt mid-trial
- Protocol review consultancy to adapt the protocol to reduce drug wastage
- Ability to pool medication across studies, aligning with program-level medication management initiatives
Improve data quality
The quality of data collection can be achieved in several ways with Calyx IRT:
- Reducing ambiguity during data capture through accurate questions and quality translations
- Removing risk of errors through data entry validation in real time
- Integrating with eClinical systems to reduce the need for data duplication
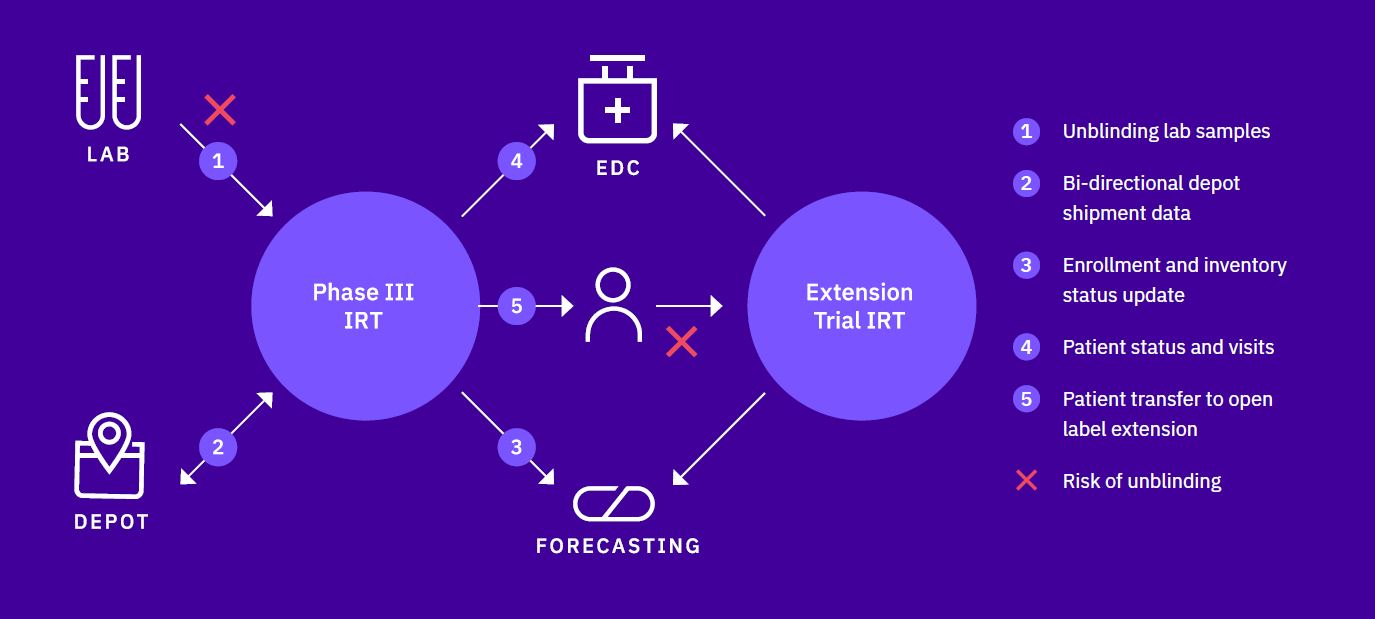
The latter also provides a reduction in data entry by site users and a reduction in need for data reconciliation at the end of the trial. At Calyx, we are extremely experienced in integrating with other eClinical systems, whether they are simple IRT-to- EDC integrations or more complex, blinded central lab integrations.
HIGHLIGHTS
- More than 10,000 files successfully exchanged with other systems every day
- Standard integrations with top EDC systems
- Standard integrations with key supply distribution providers
- Extensive experience in complex integrations, such as blinded lab data and electronic patient reported outcome (ePRO) scoring
- Ability to build integrations with new systems at trial level
- Existing processes for integration queries management through Calyx service desk
IRT integrations increase trial team and site efficiency and reduce the risk of unblinding