Overview
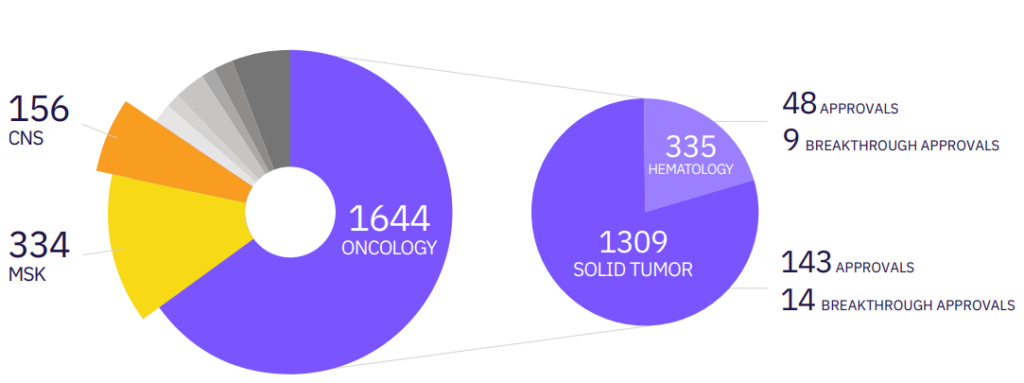
Calyx Medical Imaging is a leading imaging core lab with experience and proven capabilities in neuroimaging for clinical trials. Calyx Medical Imaging’s Central Nervous System (CNS) group understands the unique imaging needs of Alzheimer’s Disease (AD) trials and has the expertise and flexibility needed to effectively manage and scale these important clinical development programs covering early to late phases.
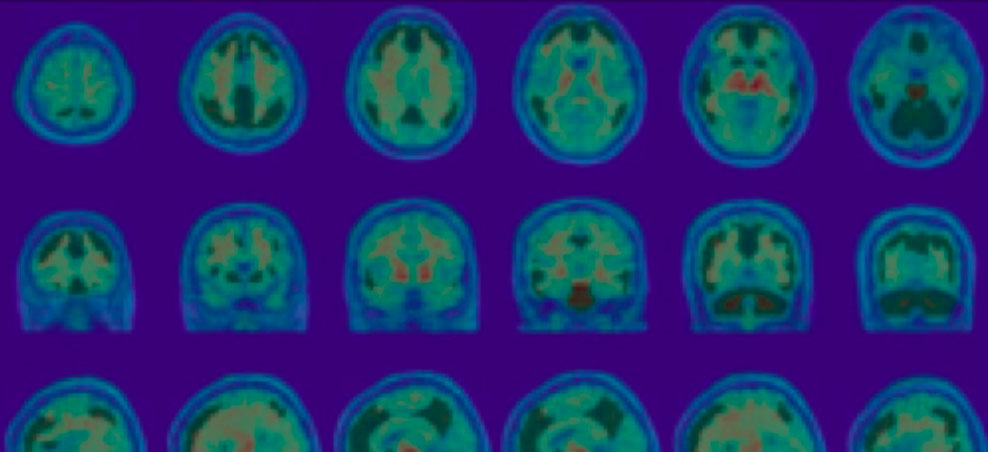
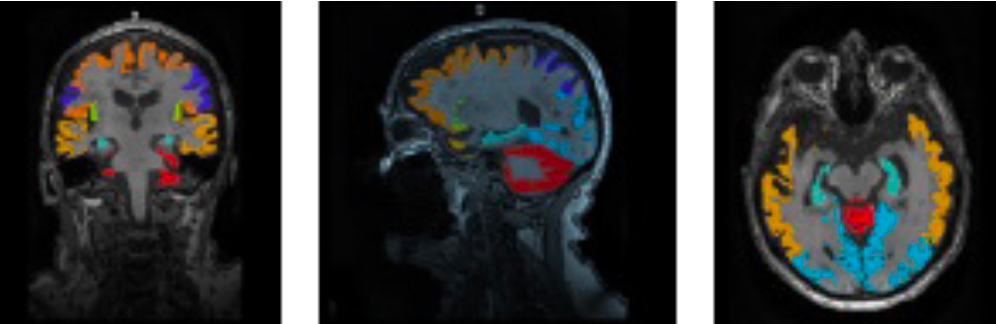
Calyx’s experience in AD includes confirmation of inclusion criteria (eligibility) and brain safety assessments throughout the study with rapid turn-around-times as well as advanced quantitative analyses for Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) data.