How does IRT help reduce drug wastage?
Interactive response technology (IRT) is a randomization and trial supply management (RTSM) tool that is often under-used when it comes to waste reduction. But when planned for early – during medication calculation and packaging planning – can be very effective at reducing drug waste and overall trial costs.
Here we review eight approaches to drug waste reduction that can be implemented through a well-designed IRT system. Since the scenarios that determine the optimal approach will vary from trial to trial, it’s recommended that clinical trial sponsors and CROs work closely with their IRT provider to leverage their expertise and take full advantage of the system’s benefits.
8 IRT Approaches That Help Reduce Drug Wastage
1) Prediction
The traditional way of supplying sites with medication is based on a buffer strategy, conventionally configured by trigger and resupply levels that can be adjusted per site. The IRT system generates a shipment request each time a resupply trigger is met for any of the pack types at site. It is common to replenish all pack types at the point a shipment request is raised to reduce overall shipment costs and to better maintain the blind within a shipment. The resupply level is a function of the recruitment rate, desired resupply frequency, site storage capacity, and study overage, and should be sufficient to supply the anticipated number of unexpected events requiring medication until the next shipment should be raised.
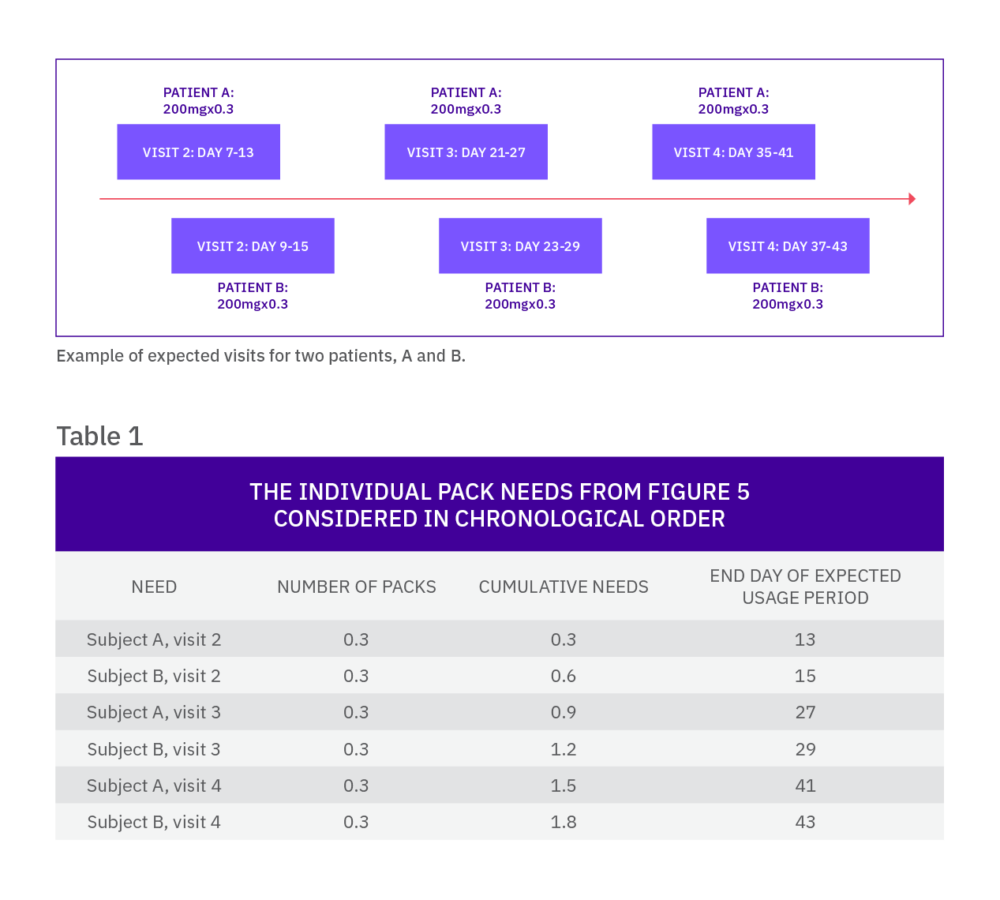
Once a subject is randomized into the trial, he or she may require additional medication dispensations, which are usually supplied at further scheduled visits. As this schedule is known by the IRT, the system can calculate which packs are required by the subject and when, which means the needs of a continuing subject can be predicted.
The IRT system looks ahead over a defined time horizon (check range) and determines if the current stock on-site is sufficient to supply any visits in this window. If it is not, a new shipment of medication must be sent to supply the unfilled requirements. To reduce the number of shipments to sites, the system looks ahead over a longer time horizon (restock range) to decide if there are any additional scheduled visits for returning subjects that can be supplied at the same time. The restock range is determined by desired resupply frequency, site storage, and study overage, alongside other factors such as expected withdrawal rate.
Although these methods of resupplying sites significantly reduce the amount of wastage compared with the traditional system of supplying subject-numbered packs in a single shipment, there are times when these methods lack the sophistication and adaptability needed for more complex scenarios.
2) Fractional-prediction algorithm
In conventional dose-finding studies, where the sponsor investigates several doses of the same treatment, reducing drug wastage becomes more challenging.
Without an IRT system, the sponsor may need to maintain all dosing options within the protocol for all patients, subsequently leading to significant wastage, particularly if there are many doses. Although a prediction algorithm in IRT will provide significant savings, it may not sufficiently cover the potential for intra-patient dose titrations which may result in the need to maintain a higher stock of medication at each site to cover for that possibility.
Consider an example
Imagine there are four doses of an active compound (and matching placebo doses) and any subject can titrate one dose level at each visit. With a simple buffer strategy, the IRT system would need to keep a pack of each dose level for each treatment on site for every patient who could expect to titrate (based on the recruitment rate).
A more efficient option may be to predict all possible doses that each patient may need for each visit (e.g. for a subject on dose level 2 at the previous visit, the system could predict one of each of dose levels 1, 2, and 3 of the relevant treatment groups).
This method, while minimizing the irrelevant treatment types kept on site, still means that three packs are being sent to the investigative site when only one is expected to be used.
There is a type of predictive algorithm that means this potential wastage can further be reduced. Based on the approximate percentage titration rates, the IRT system can predict a fraction of a pack relative to that expectation. For example, if only 20% of patients on dose level 2 are expected to down-titrate to dose level 1, one fifth of a pack can be predicted for each patient currently on dose level 2.
When the needs are assessed by the IRT system, the fractions (for each of the pack types) are added and rounded up to the nearest whole. In our example, for every five subjects on dose level 2 of the same treatment, one pack of dose level 1 will be sent to the site.
With this approach, the fraction that is predicted is an important variable: setting the fraction too high can increase drug wastage, while setting it too low could result in dispensing failures. By using drug demand simulation, the fraction can be optimized by ensuring the risk of failures is low while keeping the minimum amount of medication at site.
3) Forcing
Forcing as part of trial design
Forcing is usually used as a method to avoid randomization failure due to unplanned lack of medication at site. There are occasions where forcing in IRT is more intentionally incorporated into the design to reduce drug wastage.
Forcing at site using a double randomization.
This method is suitable in trials where there are many treatments, scarce supplies and relatively low recruitment rates. In such situations, sending a full set of treatment supplies represents a substantial amount of wastage.
To reduce wastage, two separate randomization lists are employed. A first randomization list is prepared using the smallest block size, which will be the sum of the allocation ratios. For example, a block size of seven will be used for a trial of seven treatments with an equal allocation ratio. As sites are activated in IRT, they are sent supplies corresponding to a fraction of the block size. In our example, the first site activated may be sent treatments corresponding to the first three entries on the list. The second site activated would be sent the next three entries on the randomization list and so on. This means that a site could be sent two packs of the same treatment drawn from separate blocks.
Where that first randomization list is used to determine which treatments are shipped to sites, a second randomization list is used for the actual patient randomization. That list uses forcing to allocate the patient the next available randomization number corresponding to a treatment that is available at site. Randomization numbers corresponding to non-available treatments are skipped but are available for subsequent patients. As patients are recruited at a site, buffer stock resupplies are initiated using the first randomization list and prediction is used for future patient visits.
By forcing allocation from a balanced list, this technique will result in obtaining the best overall study treatment group balance in the face of limited supplies and a minimal wastage of medication. It is worth noting that this method is not recommended by ICH E9 Guidance. Arguably though, the protection against predictability and selection bias is increased by this method as, even if investigators know the block size and past treatment allocations, they cannot predict the medication for the next patient to be randomized.
4) Automated supply strategy management
Conventional stock management based on expected recruitment rates is limited because it requires active monitoring and action from the clinical supplies team to adapt to actual site-level recruitment.
Although this a valid solution for relatively small studies, it is not relevant to expect continuous close monitoring of all sites’ recruitment rate on a large global study.
An IRT system can help with adapting the stock needs based on usage rates, which is directly correlated to actual recruitment rate.
The stock at site is still categorized by low, medium and high recruitment, but the inventory management algorithm embedded in the IRT monitors the number of patients actively on-going treatment at each site and translates it into an amount of medication required over a pre-defined amount of time.
Advanced IRT systems include the ability to work on average usage as well as highest expected values, to increase the accuracy of the supply management. Should the number of active patients at site increase or decrease, the IRT system will automatically adapt the needs at site, resulting in medication stock being automatically managed.
Such strategies can also be automatically adapted to recruitment phases if medication requirements per phase vary. Clinical supplies managers will want to reduce the buffer stock at site to the minimum when all patients have been recruited for example, which can be automatically done by the IRT, saving the need to closely monitor recruitment phases.

Should the number of active patients at site increase or decrease, the IRT system will automatically adapt the needs at site, resulting in medication stock being automatically managed.
5) Randomization prediction
Buffer stock defined in terms of trigger and resupply or usage rates are determined in terms of the number of packs that may be required over a specified period, based on an expected recruitment rate and the likely treatment allocations. Supplying medication in this way will inevitably lead to wastage as the number of packs that need to be maintained to randomize a set number of patients will always exceed their actual needs.
In a study with three treatment groups where each group receives a different medication type, to enable the randomization of two patients within a short period, two packs of each medication type must be kept on-site.
In clinical trials where the randomization code is stratified at a site level, there is a method of predicting the buffer needed by using a strategy known as randomization prediction.
The medication needs of a site are determined by the randomization schedule; by looking forward in the schedule, the IRT system forecasts future treatment allocations. Supply orders are generated for sites when the inventory levels fall below or equal to that required to randomize the next X patients to the treatments listed in the randomization schedule, where X is determined by the highest expected recruitment in the check range.
The resupply amounts are similarly determined by the randomization schedule, and shipments will contain enough medication to enable the site to randomize the next Y patients. Similar to normal buffer, Y is determined by the average anticipated recruitment rate in the restock range.
Using normal buffer, in the example above six medication packs would need to be maintained to randomize two subjects; if the randomization scheme was stratified by site and the method of randomization prediction used to maintain buffer stock for randomizations, only two packs would need to be kept onsite to enable randomization of the two patients.
6) Blinded group ordering
In many studies it is often the case that recruitment is slow, or very few subjects are expected to be recruited at each site. In these situations where the trial design is double blind, it is very likely that a shipment would contain medication for a single patient and require that a random pack is added to the shipment so that it remains blinded. This additional pack may result in a relatively large amount of wastage when considering it across a full study, particularly where there are several treatment groups or packaging types.
An alternative method of supplying buffer medication, known as blinded group ordering, can be used in this instance to reduce this wastage.
The principle behind blinded group ordering is that the initial supply to site contains the random blinding medication, rather than having to add it to each consignment. It is like a regular buffer strategy; however, the blind group ordering strategy also considers maintaining a total buffer based on an overall quantity.
For example, consider a study with two pack types, active and placebo: the blind group ordering strategy could be set to maintain one pack of active, one pack of placebo, and three packs overall. The third pack would be randomly selected (i.e. either active or placebo). Let’s assume the current stock at a site is 1 × A and 2 × P (where the second placebo is the random pack).
When the next patient is randomized, one of the following scenarios could occur:
- If the patient is randomized to active, a single pack shipment would be raised containing one pack of active (as there would be no active packs left at the site).
- If the patient is randomized to placebo, a single pack shipment would be raised containing either a pack of active or a pack of placebo (as there would still be a pack of placebo remaining at the site, the IRT system would replace the random pack).
Using such a method means that single pack shipments are not partially unblinding as the investigator does not know whether the pack in the shipment is replacing the pack they have just dispensed or whether it is a random pack. This can therefore reduce the amount of drug wastage that would otherwise result from adding a significant number of packs to blind each shipment.
7) Pack substitution (for open label studies vs double-blind)
Pack substitution in a clinical trial is usually used when a medication dispensation plan allows for multiple dosing formulations of pack combinations to be used to constitute the same overall dose. For example, a 100mg dose may be made up from 4 x 25mg tablets, 2 x 50mg or 1 x 100 mg. The decision about how to provide this dose will be made to optimize multiple different objectives, which could relate to frequency of use by the patient, ease of use for the patient, availability, or a separate clinical consideration.
In a situation where the formulation of a drug is changed during a trial, there is a high risk that switching from a formulation to the other results in a lot of wastage. If the existing packs are replaced before they are fully used, all remaining packs will be wasted. It could result in waste at both site and depot level.
Pack substitution is a good solution to reduce wastage in such a situation. The switch from the old formulation to the new formulation is done on a site needs basis, with the IRT shipping the old formulation to sites as a priority and only shipping the new formulation to sites once the depot has run out of the old formulation.
From a medication dispensing point of view, the IRT system also selects the old formulation as a priority and only starts dispensing the new formulation when there is not enough of the old one for a full dispensing.
It is crucial to plan pack substitution well in a scenario like this, as the expiry date of the new formulation needs to be long enough to account for the relatively slow replacement of the old formulation. Sponsors will also want to share this process with depots and sites, so they are prepared for the new formulation replacing the old only when all the old formulation has been used.
8) Medication Pooling
A good way to reduce drug wastage is to look at harmonizing the use of medication across a program or suite of studies, which is referred to as medication pooling. Sponsors should not only achieve a more efficient usage of medication, they can also reduce study-specific supply management oversight. A well-designed IRT solution will be a critical tool to manage medication pooling correctly and help realize costs efficiencies.
Medication pooling in IRT is not new, but many study teams are still unclear about what pooling can and cannot do, and how the regulators will react to its use. Medication pooling will not apply to all programs, as there is a need to have enough overlap in same pack type requirements across protocols for it to be beneficial. Sponsors also need to consider the impact on labelling and should refer to regulations in force across all countries included in each trial, to make sure they follow the right recommendations. Having multiple labels on packs can become confusing to depots and sites, specific labelling solutions are likely to be required to reduce both confusion and risk of error.
Let’s define medication pooling
Pooling is possible when more than one protocols operating at the same depot and/or clinical sites uses the same medication. Pooling allows medication supplies to be shared across multiple trials, hence medication for each protocol is indistinguishable in content, count, and in some cases labelling.
It is common to consider medication pooling from a medication wastage reduction point of view only, but it is also an effective solution to address availability (scarcity) concerns or other restrictions that affect the supply of medication.
When considering sharing medication across trials, medication pooling can be applied at depot level or at site level; we will review both options in this document.
Depot vs site level pooling
Depot and site level pooling have different supply chain and IRT set up considerations. The level where the pooling takes place will impact on packs labelling, depot supply management, as well as site supply management. Both levels also represent different potential savings, site pooling offering the highest medication savings.
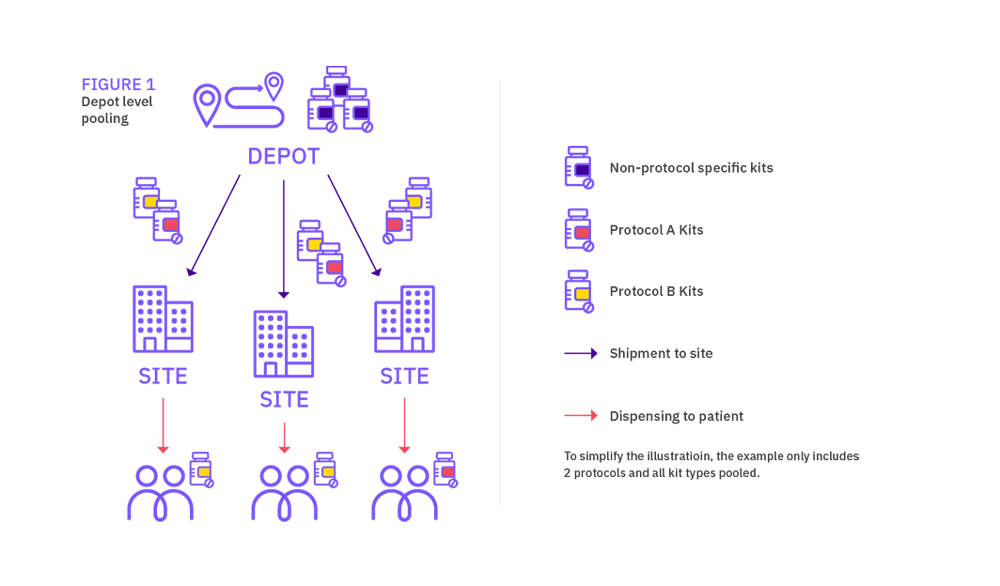
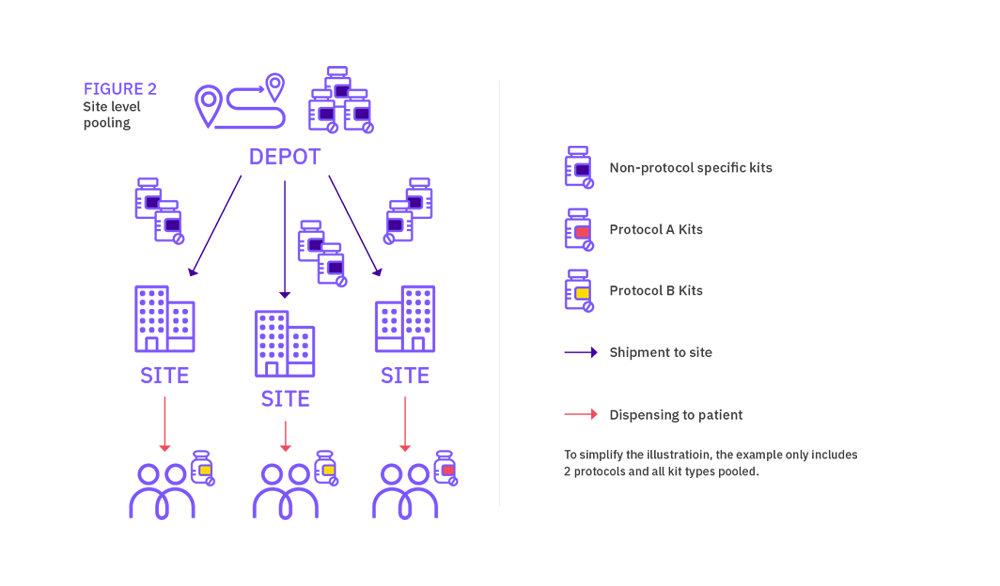
In simple terms, depot medication pooling consists in managing medication across trials at depot but having protocol-specific medication at site (even if the pack types are identical). Site medication pooling on the other hand results in managing medication across trials both at depot and site levels. The latter solution is always felt to be the best approach, however determining the size of the one pooled stock for all events in the group of studies could be complex.
Site level pooling
Although not strictly qualified as site level medication pooling, a simple way to reduce medication wastage is to add an extension protocol to the IRT designed for the existing protocol. This is common practice within the industry, and it allows to use packs across both protocols without much impact to the IRT design. This approach is limited to the combination of two protocols though.
Site level pooling associated with a program of studies allows a site to have one supply of stock for all the protocols it is recruiting into within the ‘pooling group’ of protocols. The site has one supply scheme across all the protocols, rather than a separate supply scheme per protocol. In an open label trial, it helps avoid any compliance issues where sites may run out of stock for one protocol, but have the needed medication available for another protocol.
What is the impact on site supply strategies?
Depot level pooling offers the flexibility to include both pooled pack types and medication that is not pooled, where a certain pack type is assigned to a specific protocol for example.
For site level pooling in the other hand, protocol specific packs should be restricted to medication solely used within a particular protocol for a purpose unique to that design. Sites will have one supply of medication, shipments will be raised without distinction between protocols.
Medication pooling can be used in combination with predictive supply for upcoming patient visits within a given site for all the studies in the program represented at the site. Additional buffer stock should be held to cover new patients and unscheduled resupplies. It is very important to assess needs across all protocols to determine a buffer stock that can meet the needs of the trials currently active within the site. Sponsors will also need to consider how buffer stock should be impacted if a new protocol is added to the pooling, and how to implement the right level of flexibility in supply strategies. The definition of supply strategies becomes more complex if protocols include both pooled medication and protocol-specific pack types.
REFERENCES
Sarah Waters
Calyx, Nottingham, UK
Iain Dowlman
Calyx, Nottingham, UK
Kevin Drake
Calyx, Nottingham, UK
Lee Gamble
Calyx, Nottingham, UK
Martin Lang
Calyx, Nottingham, UK
Damian McEntegart
Calyx, Nottingham, UK
Damian McEntegart
Calyx, Nottingham, UK
Proven. Adaptable. Trusted.
There’s nothing more important than keeping clinical trial patients safe. Which means ensuring they receive the right medication on time, every time.
That’s why, since 1993, the world’s leading biopharmaceutical companies and CROs have repeatedly turned to Calyx to meet their varied randomization and trial supply management RTSM) needs.
With Calyx IRT, you gain confidence in RTSM through an extremely robust system, built on decades of experience, and delivered by the most experienced project teams in the industry.
KEY FEATURES
- Advanced randomization management, suitable from simple blocked and stratified randomization designs to more complex adaptive trial designs
- Flexible and robust medication management algorithms, including automated workflows and self-service tools
- Intuitive site functionalities configured to match protocol requirements and increase protocol compliance
- Reporting adapted to the needs of each user type
KEY BENEFITS
- Reduce the risk of unblinding, randomization imbalance, or mis-dispensing by working with the most experienced teams in IRT
- Meet key milestones – Calyx teams consistently deliver excellent quality on time
- Reduce effort during IRT system setup thanks to the expertise of Calyx staff who make your decision-making process simpler
- Leverage the highly integrated IRT solution to increase data quality, reduce effort in data reconciliation, and reduce the risk of unblinding
- Decrease vendor oversight by leveraging Calyx’s excellence in project management
- Increase your confidence in supply chain settings by engaging with Calyx supply chain experts
- Reduce the cost of drug management through advanced IRT settings
Optimized through the delivery of 4,500+ trials to date, Calyx IRT is an RTSM solution you can trust. Every time.
Reduce trial risks, ensure compliance
With Calyx’s robust functionalities and proven processes, you gain confidence in your ability to control the risks of:
- Unblinding
- Imbalanced randomization
- Mis-dosing or mis-dispensing
- Supply chain disruption
Calyx IRT allows you to focus on trial execution without worrying about impacting the validity of your trial or the safety of your patients.
HIGHLIGHTS
- Central randomization list, generated by a dedicated team with experience over thousands of trials
- Statistical designers with average 7+ years’ experience in IRT and 250+ adaptive trial designs delivered
- Secure data blinding, resulting in safe navigation through Calyx IRT and safe communication with our helpdesk
- Risk of unblinding reduced to 0.0001% per transaction
- Medication management controls automatically restock sites and alert you of depot low stock, resulting in medication on site whenever patients need it
- Expiry controls that remove the risk of medication expiring in the patient’s hands
- Drug accountability, return, and destruction workflows allow you to track medication throughout its lifetime, and easily account for each single kit
Known for its ability to adapt to any clinical trial need, regardless of complexity, Calyx IRT is an advanced RTSM solution that takes the worry out of randomization and supply management so you can concentrate on study success.
Boost your productivity
With Calyx’s proven processes, you spend less time on IRT considerations and more time on trial execution.
From initiating the IRT design to database lock, Calyx’s expert project teams will recommend the best functionalities to meet your protocol requirements, selecting from our set of standards. Your time in vendor management will be reduced thanks to Calyx’s IRT expertise and broad experience managing unplanned situations. You will have access to tools that help you adapt to actual site recruitment.
HIGHLIGHTS
- Existing set of standard functionalities to select and adapt to your own standards, to further reduce IRT setup time
- As little as 4 weeks from requirements approval to UAT
- Integrations with eClinical suites (EDC, ePRO, central labs, supply management, etc.); Calyx IRT successfully exchanges more than 10,000 files with other systems daily
- User acceptance testing script writing and execution service simplifies IRT setup
- IRT-dedicated helpdesk, specialized in managing the challenges of patient and inventory management for your sites and trial teams
- Calyx supported 750+ live studies during the COVID pandemic, keeping all patients supplied with medication
- IRT self-service tools allow you to quickly adapt to varying site recruitment levels, change/add supply strategies, and activate new countries and depots
Reduce your cost of IRT ownership with Calyx IRT.
Reduce drug management costs
Calyx IRT is more than just randomization and clinical supply management solution, it also enables you to reduce the cost of drug management. Calyx project teams are experts in optimizing clinical supply chains through IRT settings. For each protocol, they assess what is most prominent between the cost of shipping to sites and the cost of drug, and recommend how to apply IRT settings that will reduce the overall cost of drug management.
HIGHLIGHTS
- Advanced IRT settings for site supply management, reducing drug wastage beyond the typical buffer stock and predictive drug shipment, such as:
- Randomization code look-ahead
- Automated recruitment-based supply adaptation
- Fractional predictive shipments
- Statistical designers with average 7+ years’ experience in IRT help you to best balance the cost of drug and cost of shipments
- Supplies management adapting to central and local sourcing strategies
- Self-service tools provided to clinical supplies management team to adapt mid-trial
- Protocol review consultancy to adapt the protocol to reduce drug wastage
- Ability to pool medication across studies, aligning with program-level medication management initiatives
Improve data quality
The quality of data collection can be achieved in several ways with Calyx IRT:
- Reducing ambiguity during data capture through accurate questions and quality translations
- Removing risk of errors through data entry validation in real time
- Integrating with eClinical systems to reduce the need for data duplication
The latter also provides a reduction in data entry by site users and a reduction in need for data reconciliation at the end of the trial. At Calyx, we are extremely experienced in integrating with other eClinical systems, whether they are simple IRT-to- EDC integrations or more complex, blinded central lab integrations.
HIGHLIGHTS
- More than 10,000 files successfully exchanged with other systems every day
- Standard integrations with top EDC systems
- Standard integrations with key supply distribution providers
- Extensive experience in complex integrations, such as blinded lab data and electronic patient reported outcome (ePRO) scoring
- Ability to build integrations with new systems at trial level
- Existing processes for integration queries management through Calyx service desk
IRT integrations increase trial team and site efficiency and reduce the risk of unblinding
Learn how IRT consulting and design from Calyx can support platform trials to accelerate the clinical development lifecycle.
This case study reviews the importance of shipping medication directly to clinical trial patients in order to minimize late dispensing visits and keep patients treated and safe during COVID-19 and beyond.
This case study presents how Calyx IRT can successfully manage various sourcing strategies including the ability to adapt to centrally and locally sourced medications and switch sourcing requirements during the course of a trial.
Optimizing integrations with Calyx’s industry-leading IRT system reduces data entry and increases quality in clinical trials. Learn how different sponsors have benefited in this case study.












