In advance of his presentation at the Global Clinical Supplies Group Conference, Calyx’s Sylvain Berthelot reviews how effective IRT solutions can have a big impact on reducing drug wastage for more efficient and cost-effective clinical trials.
Introduction
A large proportion of the cost of implementing a clinical trial is attributable to the production and management of medication. Over the past 25 years we have seen the management of medication in clinical trials move from a manual process to automated algorithms and workflows, leveraging IRT for Randomization and Trial Supply Management (RTSM) functionalities.
As IRT usage became more widely included, even in non-randomized and open-label clinical trials, it became a support for drug wastage reduction. By automating the trial supply chain, the available medication is directed to sites that are actively recruiting or treating subjects, thus ensuring those sites have sufficient stock to supply their subjects, while at the same time limiting the quantity of medication wasted by overstocking unproductive sites.
Here, we present a backgrounder on how effective IRT solutions can have a big impact on reducing drug wastage for more efficient and cost-effective clinical trials.

“What we intend to do by reducing waste in a clinical trial is identify methods upfront to reduce the quantity of drug that needs to be manufactured, stored and shipped to sites.”
– Sylvain Berthelot, Calyx
Defining drug wastage
It is important to define what we mean by drug wastage, as not all kits unused by the end of a trial have been wasted. In double-blind clinical trials, where sites and patients do not know which treatment is being dispensed, kits part of the site’s stock are needed to perform medication checks that are required to remove the risk of unblinding and to ensure randomization can be performed. It is worth noting that these kits are not wasted, as randomization or dispensing would not occur without the presence of all kit types at site, even in random scenarios where all patients at one site are part of the same treatment group.
Situations increasing drug wastage
Some trials will be more prone to increased drug wastage than others. For example, a single-country trial with only a handful of sites will result in less waste than multi-country trials supported by a complex supply chain. It is also worth noting that trials where kits are inexpensive will not require specific focus on waste reduction, as other supply chain factors will be more expensive than the drug cost such as cost of shipments.
Trials where we tend to see the most drug wastage include those with a lot of treatment arms, where the need to have medication suitable for all treatment arms at site is a source of waste. Trials where the same drug can have multiple formulations, or several dosing options may also be subject to a consequent amount of waste.
Click here for a quick glance at the various factors that drive drug wastage and the different IRT approaches that can be used to reduce each.
Proven IRT approaches to reducing drug waste
Most trial supply managers are familiar with the common supply scheme methods of Buffer, Trigger Resupply, and Prediction. These enable us to prepare for events that we can’t predict, such as new patients randomized/enrolled or kit replacement. And once subjects are randomized and in the IRT system, these approaches take advantage of knowing visit schedules and what medication is needed for dispensing visits, which optimizes study drug in a way that’s expected.
Supply schemes in IRT, the tip of the iceberg
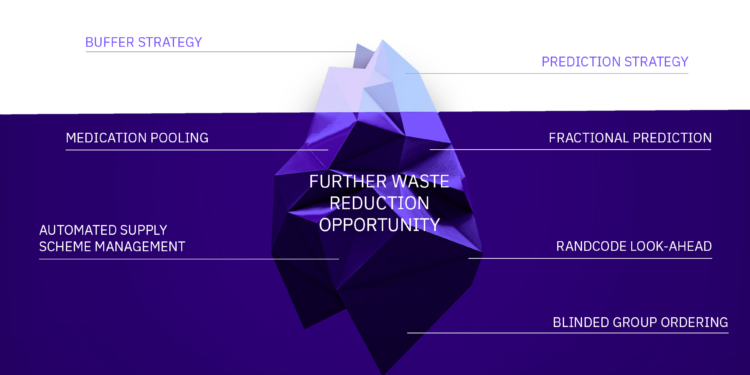
But these commonly used waste reduction approaches are just the tip of the iceberg. Underneath the water line, IRT provides additional options such as Fractional Prediction, Automated Supply Scheme Management, Rand Code Lookahead, and many others (figure 1). Each has different applications in reducing drug wastage based on different protocol designs, supply chain structure, and complexities.
Figure 1: The optimal IRT approach for reducing drug wastage depends on protocol design, supply chain structure, and complexity
Conclusion
Today’s IRT solutions offer numerous approaches to reducing drug wastage during clinical trials. To fully realize the positive impact those solutions have on drug wastage, sponsors should understand how they affect their manufacturing quantities during the trial planning. Otherwise medication is wasted at the central depot if already labelled for the protocol.
In the future, we expect additional ways to reduce wastage by expanding the use of just in time labelling. The emergence of smart packaging also provides more flexibility to clinical supplies management, simplifying medication pooling with the potential to adapt the label up to the time of dispensing.
















