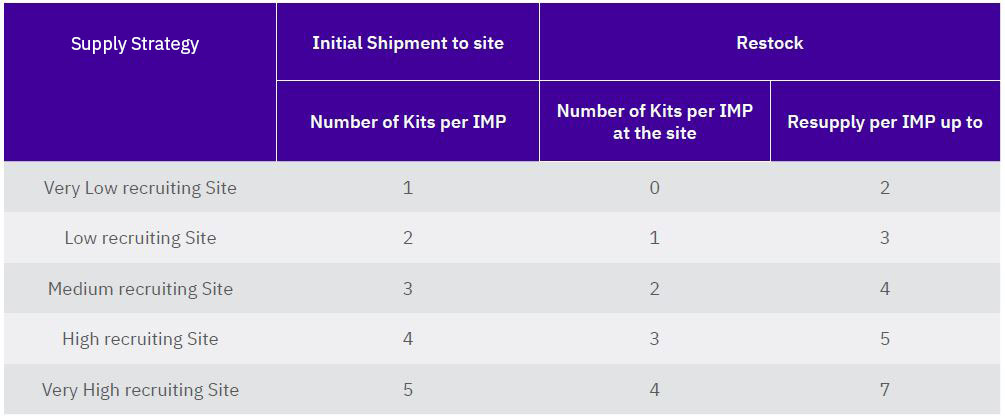
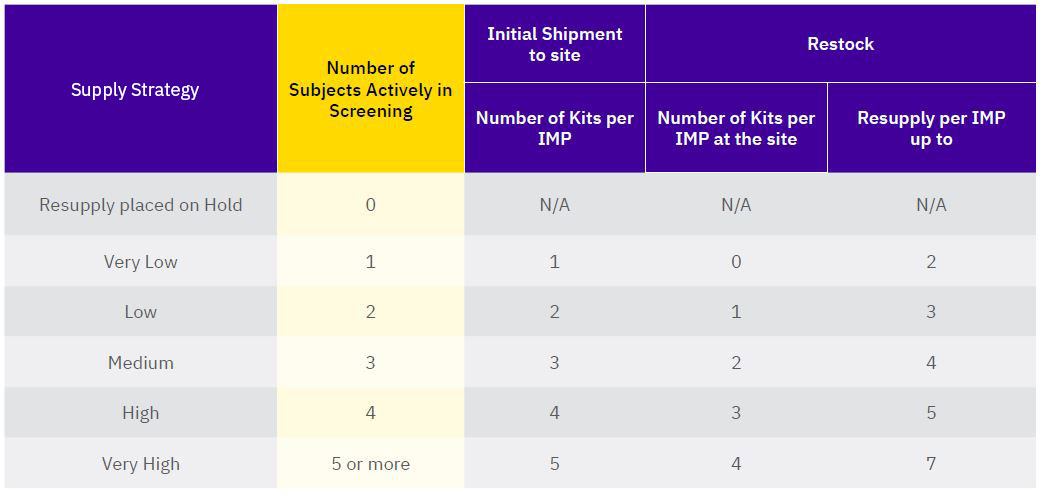
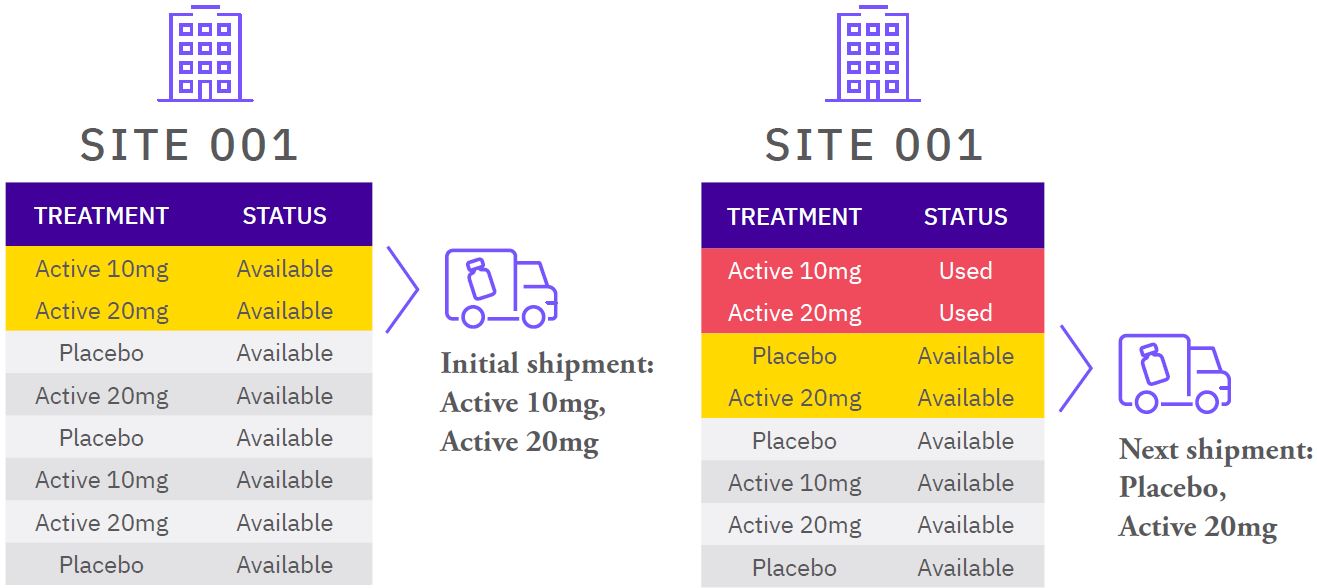
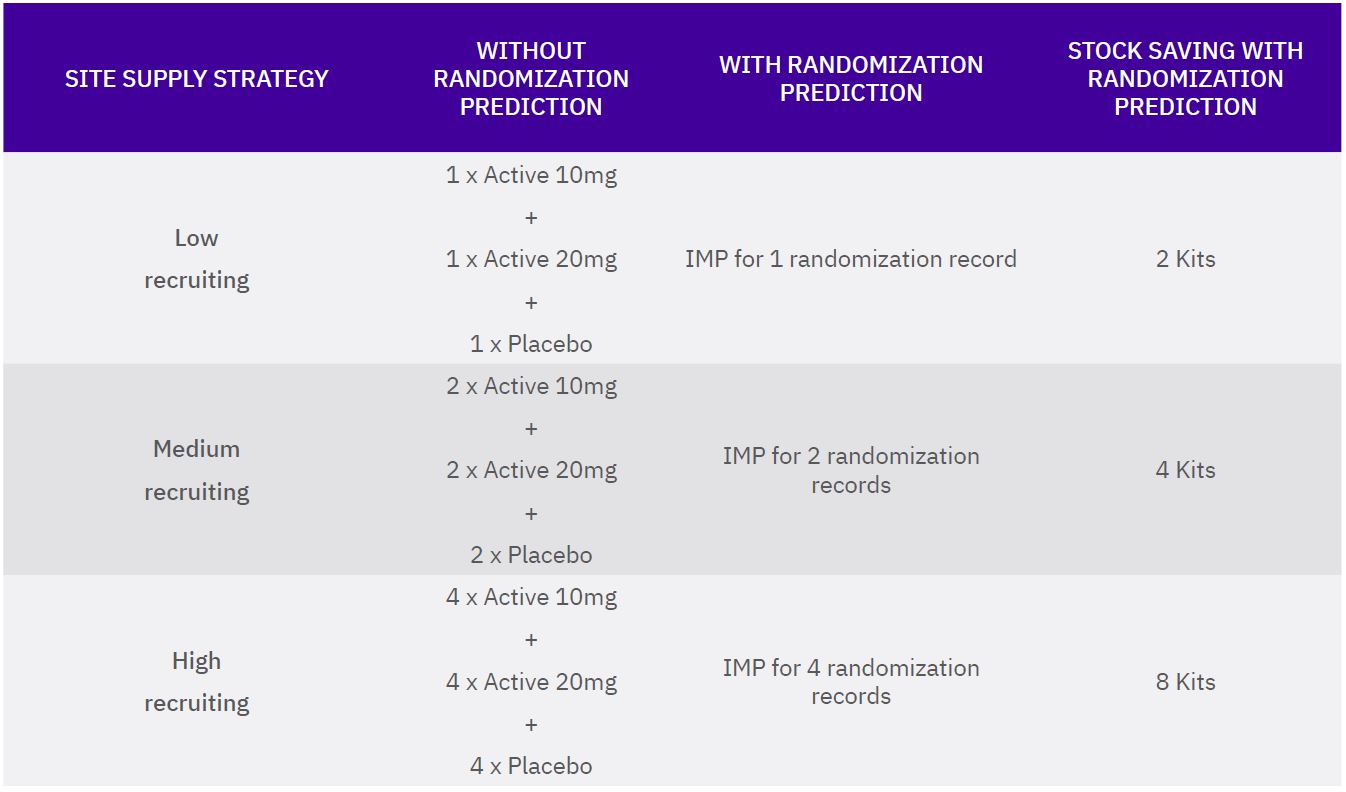
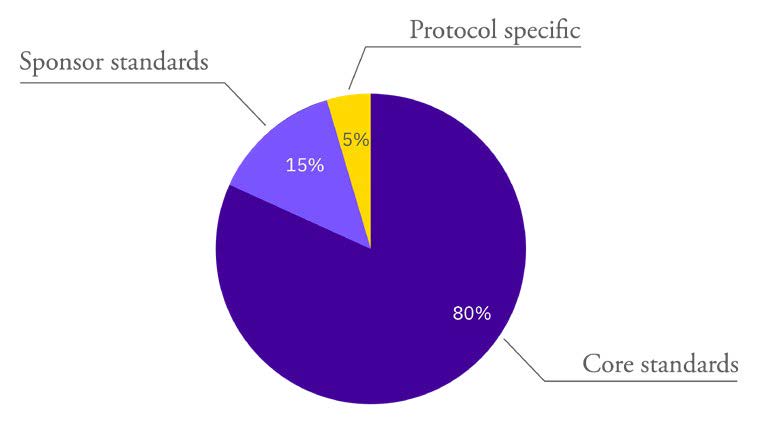
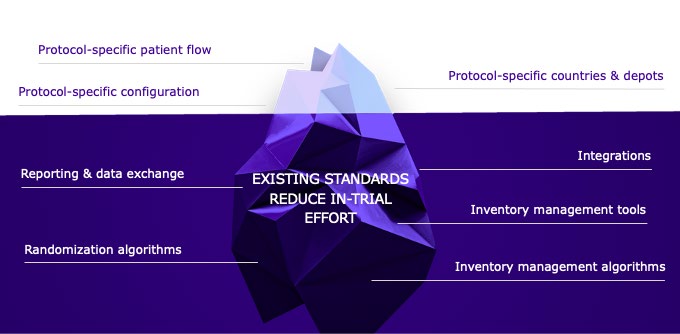
BACKGROUND
A leading pharmaceutical company selected Calyx IRT to provide randomization and trial supply management (RTSM) services for two Phase III, multi-country studies. During trial execution, a safety regulation was issued that required the company to modify its primary packaging due to safety risk concerns for some patients.
This unexpected study change was complicated in that it only affected sites in a single country and each trial had at least 12 countries participating.
At the time, the inventory was already in use across all sites and all countries, with several hundred patients enrolled and randomized, and the trials were barely at the halfway mark.
The client came to our team with a date when the new inventory needed to start being dispensed for patient visits based on the regulatory requirements. So, the experts behind Calyx IRT went to work.