The landscape of imaging in Myeloma patients has changed rapidly over the past few years. Routine clinical practice and clinical trial settings now utilize more advanced imaging modalities like PET-CT, whole-body MRI, and low-dose CT as compared to whole-body skeletal surveys (Xray) which were more commonly used in the past. These high-resolution imaging modalities lead to higher lesion detection rates at screening and thereby require an ongoing imaging evaluation in a predefined manner to support the efficacy analysis of a therapeutic intervention in clinical trials.
The figures below illustrate imaging presentation in myeloma patients and provide guidance on categorizing these lesions for response evaluation:
Going to ASCO 2024?
Meet Calyx’s Medical Imaging Experts at Booth 10098
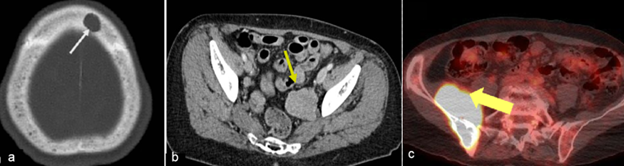
Figure a) is a CT head of a patient showing a punched-out lytic lesion in the frontal bone (white arrow). Lytic lesions should be monitored qualitatively as per clinical discretion for progressive disease.
Soft tissue plasmacytoma noted in the pelvis (yellow arrows) in two different subjects: contrast-enhanced CT of pelvis (figure b) and a fused FDG PET image (figure c). Such soft tissue plasmacytoma need to be quantitatively evaluated at a predefined imaging schedule to evaluate the response of therapy along with the other lab parameters.
Regulators are looking for standardized methodologies to be applied upfront in trials to ensure the robustness of the data and its validity. We have noted a trend in the last year wherein health authorities across the globe are asking for a central review of imaging evaluation to mitigate variability in data from site imaging interpretation and across trials.
Choose Medical Imaging.
Learn how Calyx helped a leading pharmaceutical company receive accelerated approval for a Multiple Myeloma treatment.
Calyx is a leading provider of myeloma trial imaging, having supported nearly 40 trials and 6 approved indications to date. We routinely collaborate with myeloma trial sponsors and consult on how to standardize image acquisition and support independent, harmonized imaging analysis to meet regulators’ expectations.
Our expert methodology is based on the wealth of experience gained by supporting trials over the last 25 years and engaging in scientific consultation with experts such as Dr. Shaji Kumar and Dr. Joseph Mikhael.
Contact us to explore how our scientific and medical imaging experts can partner with your team to reduce risks and deliver reliable imaging data that meets regulators’ requests and sets your development program up for success.