In this second installment to the “Simplifying the Complex” blog series, Calyx’s Malcolm Morrissey presents the factors to consider when selecting an IRT provider, and how these systems can be instrumental in reducing risks linked to randomization and trial supply management (RTSM) in adaptive trials.
Adaptive Trial Designs
The US Food and Drug Administration (FDA) defines adaptive trials as those that “allow the trial to adjust to information that was not available when the trial began.”[1]
This adaptability presents challenges for the accompanying IRT system, as it is impossible to know at the outset of the trial what changes may ultimately occur. For example, in a dose-finding study, one may assume that some treatment arms, or dose levels, may close (for futility) while others may open. However, until the data from an interim analysis is available, one cannot know exactly which arms will be affected, or which new doses will be investigated. (See Fig 1.)

“The IRT system should be highly configurable and the provider able to foresee the type of adaptations that will likely arise during adaptive trials.”
– Malcolm Morrissey, Calyx
Randomization / Drug Assignment
When an arm is closed, the IRT system must immediately reflect the decision to avoid recruiting more patients in the futile arm. The randomization algorithm must be intelligent enough to know which treatment arms are open at any point in time.
From a system perspective, adding a treatment arm is more complicated than closing one, and again, it must be done quickly. Because this involves rewriting the randomization list or finding ways to expand it so that the randomization algorithm takes into account the new treatment arm, the change will require reconfiguring (and possibly reprogramming) the system.
When a clear, closed set of adaptations are defined in the protocol, a randomization list adapted to each scenario can be prepared in advance. Unfortunately, when the protocol allows for an open set of adaptations, it is impossible to prepare for future changes. Many, but by no means all, adaptive studies may well include a closed set of options. Bayesian response adaptive studies and platform trials illustrate the need for an IRT to cater to any eventuality.
Supply Management
When an arm is closed, the medication supply algorithm will have to be updated to reflect the doses that will no longer be needed. For the benefit of blinded staff, the new supply scheme may need to maintain the illusion that nothing has changed. If the design is suitably structured, it may be that kits earmarked for one arm can be used for another. For instance, kits originally intended for an arm receiving a 25mg dose may be doubled up for use in a newly opened arm receiving a 50mg dose. Thus, in this situation, the supply algorithm within the IRT must be able to substitute one kit type with another, or a combination of kit types.
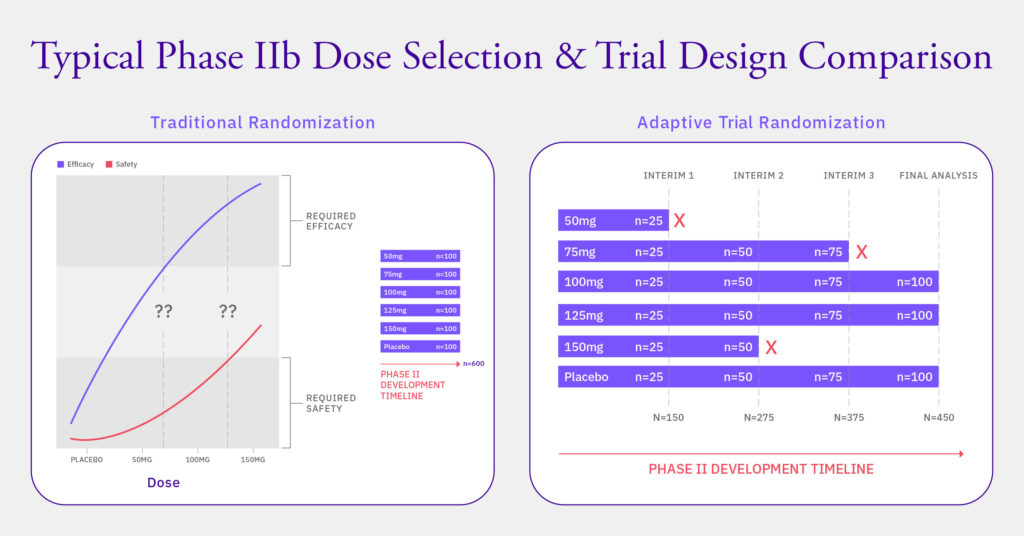
Fig. 1: Difference between traditional and adaptive trial designs and the positive impact on patient population
Three Steps to Effective Randomization and Supply Management
To ensure that sponsors realize the full benefits of an adaptive trial design, they should:
- Select an IRT vendor with demonstrable experience in supporting adaptive trials and in building flexible systems. There are unique elements to adaptive trials, and experience with other trial types isn’t necessarily transferrable.
- Look for significant, in-house statistical expertise in the IRT provider. This is necessary to ensure that randomizations will remain balanced across treatment arms, even as they change. Without the right statistical input, the validity of a trial could be jeopardized.
- Keep the vendor appraised of the status of their analyses and assessments. Ideally, the sponsor should consider the IRT vendor as an extension of the clinical trial team and maintain strong communications with the IRT partner.
In Summary
In adaptive trials, the choice of an IRT provider should be made carefully, and with a full understanding of what will be required for success. The system should be highly configurable and the provider able to foresee the type of adaptations that will likely arise. Sponsors should be able to leverage the experience and processes of their IRT partner to run these types of trials smoothly.
















