Minimizing the risk of unblinding during clinical trials is of paramount importance. Clinical trial sponsors, monitors, investigative site personnel and all others involved have a responsibility to ensure that unintentional unblinding does not occur.
In an earlier blog we established the difference between partial and full unblinding. Here we review the trial trigger points that may result in unintentional unblinding including scenarios during randomization, dispensing, shipping, site supply, drug accountability, and study management.

“It’s important to work with a reliable IRT provider who understands your study needs and will design a system to mitigate the risk of unintentional unblinding at every stage.”
– Malcolm Morrissey, Assoc. Director, Statistics, Calyx
Hear from the expert! In this webinar, Calyx’s Malcolm Morrisey presents “Unintentional Unblinding: Don’t Miss Hidden Risks.”
Randomization
- Reporting randomization number when blocks are dynamically allocated to strata (with a higher risk with site stratification)
- Reporting randomization number when forced randomization is permitted (with a higher risk with site stratification & if there are only 2 treatment arms)
- What IMP the IRT system checks is available at site before randomization is permitted to proceed (the risk depends on the number of treatment arms & also whether forcing is permitted)
- Replacing patients at the end of the cohort
- Reporting randomization number when patients are being replaced
- If re-randomization is required for a sub-set of treatment arms
Dispensing
- What IMP the IRT system checks is available at site before medication assignment is permitted to proceed (the risk depends on the number of treatment arms)
- Selecting which kits should be dispensed to patients
- When dispensing only a partial amount of required treatment (which could be allowed if the patient can be given sufficient medication until the remainder can be provided)
- When blinded medication is used in an open label manner (i.e., single blind run-in)
- When the dose level is known to Investigators, but the treatment is not
- When one or more treatments are supplied by the site (i.e., the IRT only manages the ‘active’ kit, with the placebo supplied by site: local & central sourcing)
- If third party lab data is required to make a patient treatment decision (see figure)
Shipping
- Selecting which kits should be included in shipments
- If a partial shipment is sent to a site due to a stock out at a depot
- When shipping in boxes, but dispensing individual packs to patients (the risk depends on the number of treatments in a box, whether dispensation is “random” & also whether forcing is permitted)
- When different expiry dates are used across blinded batches
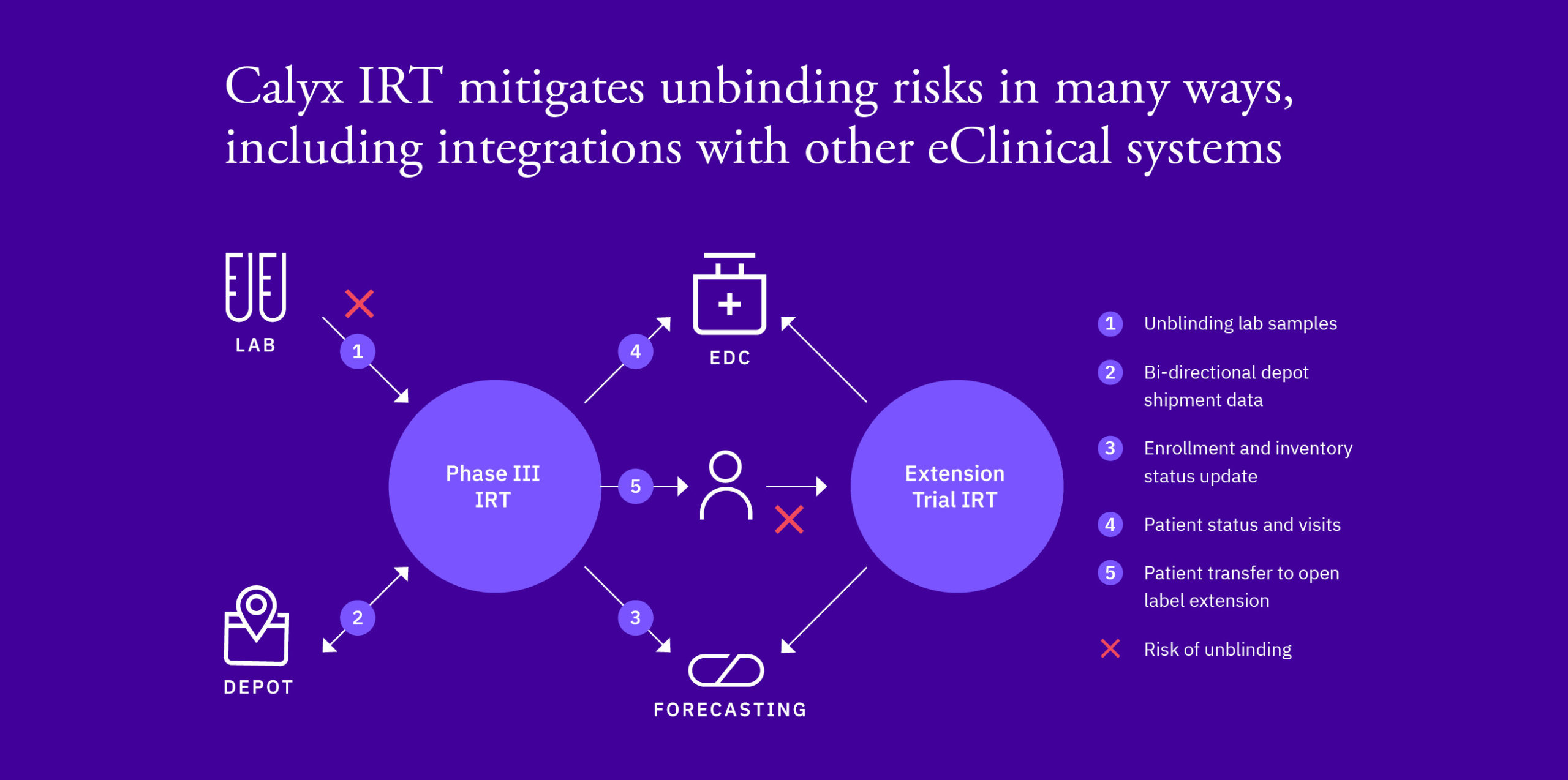
Site Supply
- If there are different trigger & resupply values (buffer stock) for different IMP
- If the initial shipment to the site only contains 1 of each IMP
- When new doses are added e.g., for an adaptive study
- If medication is predicted for all possible treatments for re-randomization when not all patients re-randomize
- If medication is predicted beyond re-randomization for patients who are not re-randomized and not all patients re-randomize
- When predicting for an open label extension where medication is not the same across treatments & is determined by blinded treatment (see figure)
Drug Accountability
- If there is variability in returns and/or destruction procedures for each blinded kit type en-route to or at the destruction facility
- If there are both blinded and unblinded site personnel, and variations in drug accountability process for different blinded kit types at site
- If expiry date is unblinding
Study Management / Data Changes
- If the study is open label, but the Study Team are blinded to treatment group
- If the study is Double blind, but there is open label treatment
- When there are two treatment groups and patients receive a different number of kits based on their treatment group
- When the IRT is only managing one treatment group
Don’t miss the last installment to this series, Clinical Trial Unblinding: What it is and what it isn’t, where Calyx’s Craig Mooney presents issues – some of which might be considered controversial – related to unintentional and partial unblinding in clinical trials.