The United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA) has announced a new route for expedited pre-and post-authorisation procedures. The International Recognition Procedure (IRP) is a new framework that will replace the EC Decision Reliance Procedure (ECDRP) and incorporate the Mutual Recognition/Decentralised Reliance Procedure (MRDCRP) starting from January 1, 2024. It aims to expedite access to safe and effective medicines for patients in the UK by recognizing trusted regulatory partners.
The IRP allows the MHRA to consider the expertise and decision-making of regulatory authorities from other countries. It will be open to applicants who have already received authorization for the same product from one of MHRA’s specified Reference Regulators (RRs – see below). The same product is defined as having the same qualitative and quantitative composition, active substances, excipients, and pharmaceutical form.
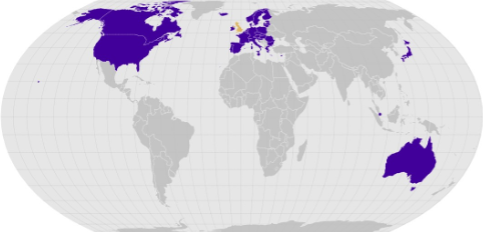
The MHRA has approved seven countries/regions as Reference Regulators; Australia, Canada, European Commission (replaces ECDRP), Japan, Switzerland, Singapore, and the USA.
The Reference Regulators play a crucial role in the IRP. Applicants seeking approval from the MHRA can apply through the IRP if they have already received authorization for the same product from one of the specified Reference Regulators. The MHRA will conduct a targeted assessment of an IRP application based on the expertise and decision-making of these Reference Regulators.
The IRP can be used for various types of marketing authorisation applications (MAAs), including chemical and biological new active substances, generic applications, hybrid applications, biosimilar applications, and new fixed combination product applications.
Traditional Herbal Registrations, Homoeopathic Registrations (Simplified Registration Scheme), Homeopathic National Rules Authorizations (National Rules Scheme), and bibliographic applications are excluded from IRP. Post-authorization procedures such as line extensions, variations, and renewals are also eligible for IRP.
There are two types of recognition procedures: Recognition A and Recognition B. Recognition A is suitable for products with overseas regulatory approvals granted within the previous 2 years and without significant complex factors. The processing window for this type of application is 60 days. Recognition B is ideal for more complex cases and has a processing window of 110 days.
The official guidance from the MHRA states that they will conduct a targeted assessment of IRP applications but retain the authority to reject applications if the evidence provided is considered insufficiently robust.
For more information about Reference Regulators and the International Recognition Procedure, you can refer to the official guidance published by the MHRA on their website.
International Recognition Procedure allows your products to reach the UK market faster for the patients that need them. All of which can be tracked and published in Calyx RIM today, using out-of-the-box functionality. With decades of regulatory and user experience, Calyx RIM can always be relied upon to support you in continuing to meet regulatory requirements worldwide.