Calyx Medical Imaging provides focused support to drive successful FDA submission
Background
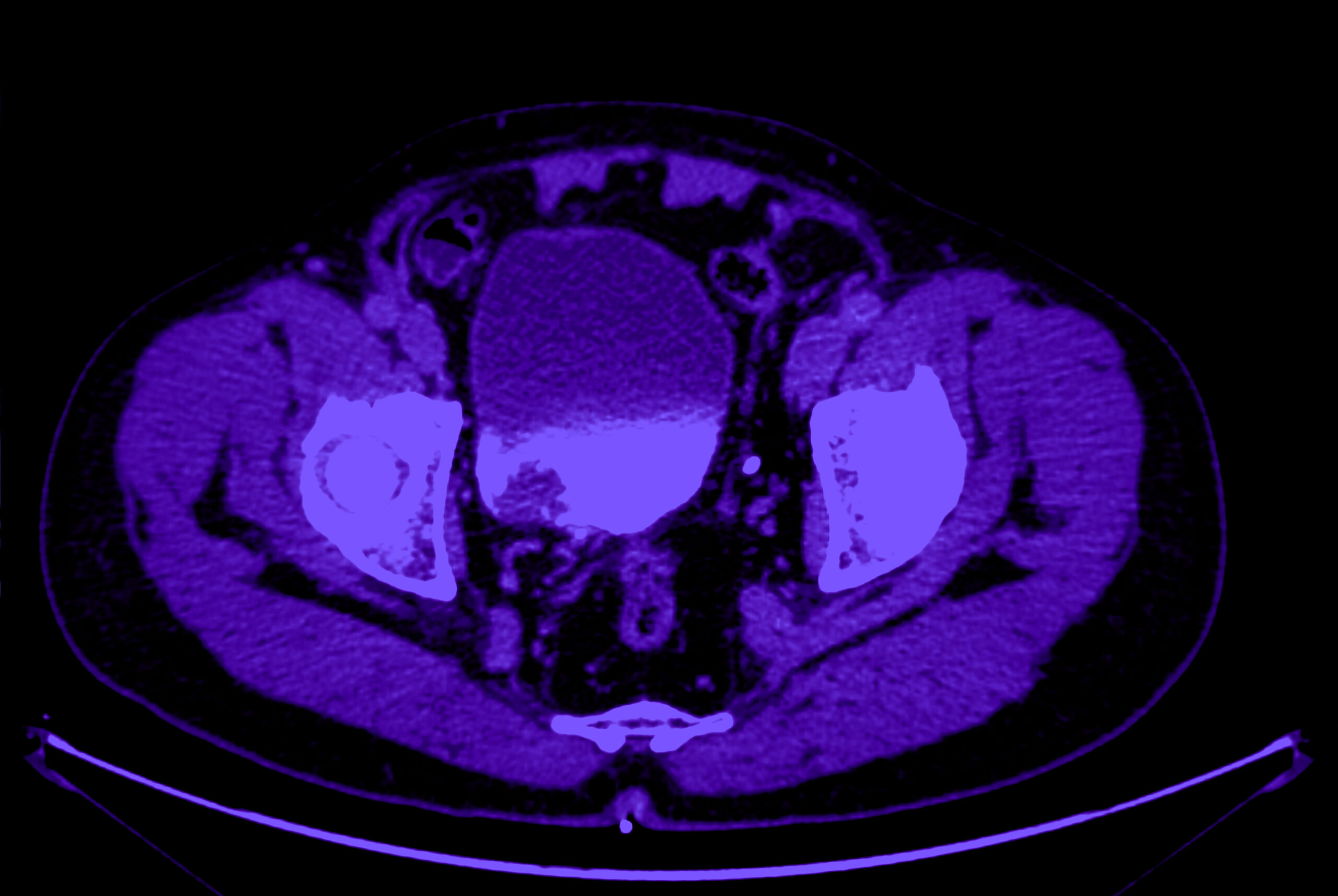
A global biotechnology company was developing a novel compound for bladder cancer and chose Calyx Medical Imaging to support its Phase 2 trial. The trial involved over 100 investigative sites across North America and Europe, capturing CT/MR images from over 400 patients to support the study’s primary efficacy endpoint.
Calyx managed all the sponsor’s imaging needs for this trial, including the drafting of an imaging charter, training of site personnel on image acquisition, and the selection, training, and oversight of a centralized imaging reviewer network comprised of five radiologists with expertise in bladder cancer assessment.
Challenge
Response assessment per RECIST for oncology trials having ORR (objective response rate) as a primary endpoint poses a challenge when subjects are enrolled with disease which may be variable in image interpretation as target disease vs non-target disease. This challenge is especially common in malignancies like bladder cancer and ovarian cancer where the tumor presentation can have inherent variability in lesion categorization as measurable vs not measurable due to lack of reproducibility in measurement on follow-up time points. This variability in lesion assessment led to high rates of discordance between site and central readers’ assessment of baseline measurable disease status, thereby impacting the endpoint of the trial.
Solution
Having supported 15 Phase 2/3 bladder cancer trials to date, Calyx’s medical team has in-depth knowledge of managing these situations. When the discordance is significant it needs to be addressed upfront and in an ongoing manner to ensure that the trial results are not impacted. An initial conversation about consideration of central review-based eligibility was proposed, however due to challenges associated this step was not implemented and enrollment was based on site assessments for this trial.
To manage this conundrum, Calyx provided case-based examples of bladder lesions that could be considered measurable vs non-measurable by the sites for enrollment based on measurability. Similar training sessions were conducted for the central readers, achieving harmonization across site and central assessments and thereby narrowing the discordance gap. There were several in-depth conversations between the medical leads at Calyx and the sponsor during the trial, ensuring compliance in response assessments per the protocol and that any potential topics that were causing variability in assessments were addressed by ongoing training.
Result
Based on this approach and the resulting efficacy data supported by Calyx’s medical imaging review process, the compound received accelerated approval by the FDA and is now presenting a new, meaningful treatment option that has the potential to address a very high unmet medical need for patients with bladder cancer.
The sponsor appreciated Calyx’s leadership and continued support through the entire process and stated that they were thrilled with Calyx’s hard work and efforts, which enabled them to submit quality data that led to the FDA’s approval.
Calyx Oncology Experience
Calyx has the industry’s most extensive experience in oncology clinical trial imaging, having successfully supported over 1,500 clinical trials across 90 unique oncology indications. This includes more than 50 pivotal studies – where imaging data supported successful submissions to regulatory agencies – and over 75 trials of compounds that were designated breakthrough therapies.