Past Webinars
Stay up to date on our latest events and webinars.

Medication supply shortages have become a widespread industry concern. However, a considered RTSM design can prevent shortages from jeopardizing your study goals.
Topics Covered: IRT
In this webinar, Professor Fendler from the University of Essen and Calyx’s Dr. Oliver Bohnsack will address these challenges and provide recommendations on how to overcome them based on their own lessons learned from conducting prostate cancer trials.
Topics Covered: Medical Imaging
Watch a quick demo of Calyx product on demand
The current lack of objective biomarkers presents a real challenge for researchers developing treatments for psychiatric and neurological diseases. But now, a partnership that combines Calyx’s clinical trial imaging expertise and Ceretype’s novel functional MRI (fMRI) platform enables trial sponsors to overcome this challenge and see the physical action of a drug on patients with psychiatric and other neurological disorders.
Topics Covered: Medical Imaging

This session will focus on the difficulty of evaluating HCC patient scans after local intervention and how sponsors can overcome the complexities associated with imaging in HCC clinical trials.
Topics Covered: Medical Imaging

In this webinar, Calyx’s Peter Tarbox shares the RTSM factors oncology trial sponsors should consider in their IRT design, focusing on how advanced IRT systems can accommodate changes in centrally and locally sourced medication for optimal clinical trial supply management.
Topics Covered: IRT
In this webinar, Calyx’s Malcolm Morrissey and Maarit Kelvin Korpos review other scenarios of how study data can lead to unintentional unblinding and the processes sponsors and their IRT solution providers should follow to mitigate these risks.
Topics Covered: IRT

Get a glimpse into what it’s like to work with Calyx Medical Imaging and how our professional client delivery teams deliver optimal imaging solutions that meet your study needs.
Topics Covered: Medical Imaging

This scientific discussion will review how emerging AI solutions that employ quantitative neuroimaging can help address some of the key challenges faced in Alzheimer’s Disease.
Topics Covered: Medical Imaging

In this webinar, Calyx’s Head of Statistics and Product Support Services, Malcolm Morrissey shares his experience on how to identify the randomization implementation options that can lead to unblinding of the block size and other potential risks that could result in selection bias at the site.
Topics Covered: IRT

Learn how the functions and features of Calyx IRT get the right treatment to the right patient at the right time, regardless of how complex your RTSM needs are.
Topics Covered: IRT

In this webinar, Dr. Oliver Bohnsack of Calyx – a leading expert on RECIST and irRECIST – discusses the implications of these findings, what it means for oncology clinical development and treatment decision-making, and why irRECIST easily can and shall replace outdated RECIST 1.1 on all solid tumor trials going forward.
Topics Covered: IRT
A panel of clinical trial sponsor and distribution partner representatives – all with experience in direct-to-patient shipments – will review what is possible to achieve and what constraints may be encountered when considering DtP for clinical trials. We’ll also hear from the most important clinical trial stakeholder, a patient who has experienced the shift from in-clinic visits to treatment delivered at their doorstep; he will share his perspective as it relates to the development of a DtP clinical trial strategy.
Topics Covered: DtP
Topics Covered: DtP

Topics Covered: Medical Imaging
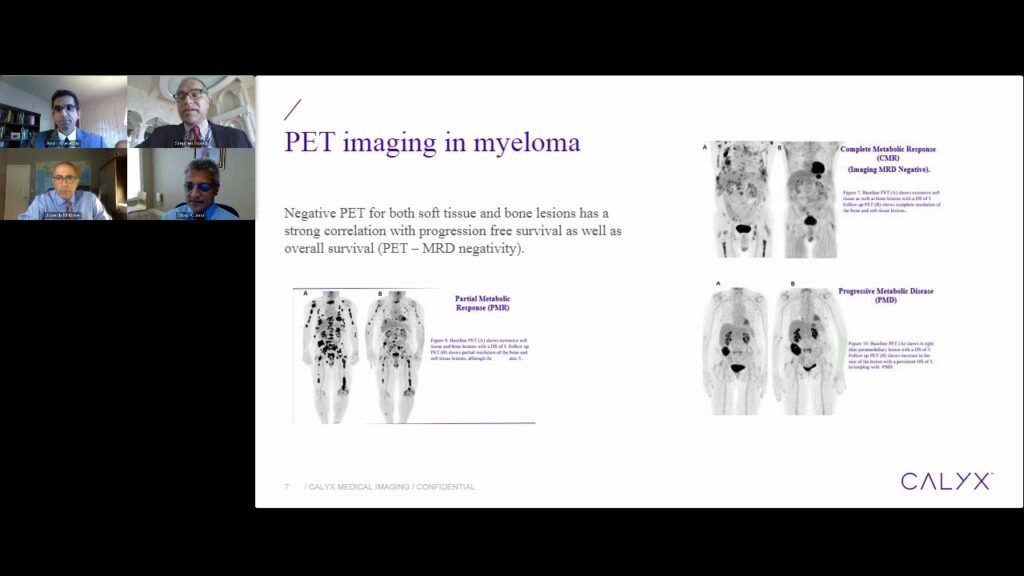
In this live virtual event, a panel of key opinion leaders including IMWG 2016 authors and imaging experts will answer your questions about the changing landscape of imaging in multiple myeloma trials and how to optimize imaging-related assessments.
Topics Covered: Medical Imaging
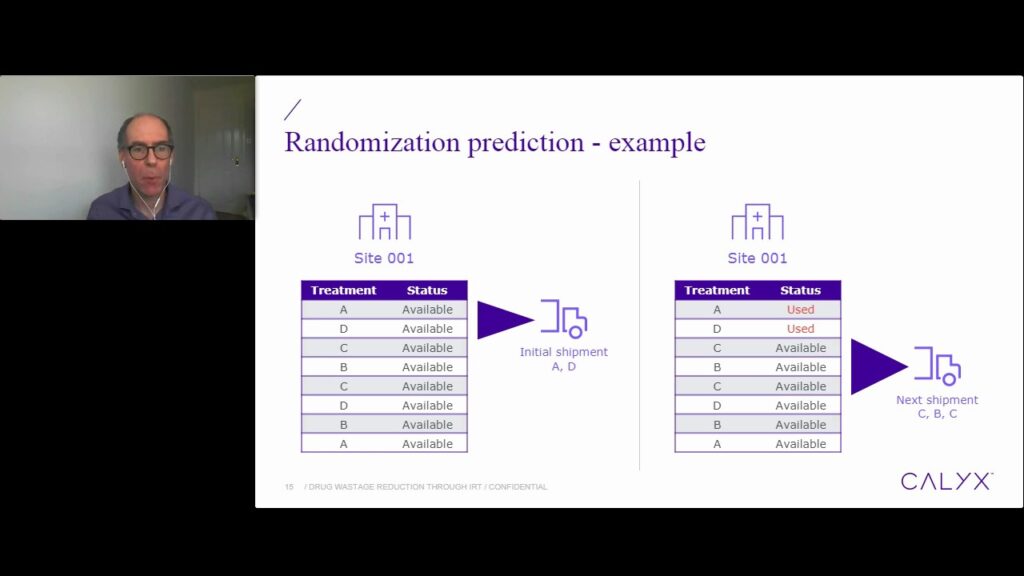
In this webinar we review how, by applying advanced IRT settings to control the supply chain, medication can be directed to recruiting sites only and amounts shipped to those sites can be reduced to the minimum, overall limiting the quantity of medication wasted at site.
Topics Covered: IRT

Join Calyx’s Head of Statistics and Product Support Services, Malcolm Morrissey, for a webinar focusing on an undervalued aspect of study and IRT design.
Topics Covered: IRT
Medication supply shortages have become a widespread industry concern. However, a considered RTSM design can prevent shortages from jeopardizing your study goals.
Topics Covered: IRT
In this webinar, Professor Fendler from the University of Essen and Calyx’s Dr. Oliver Bohnsack will address these challenges and provide recommendations on how to overcome them based on their own lessons learned from conducting prostate cancer trials.
Topics Covered: Medical Imaging
The current lack of objective biomarkers presents a real challenge for researchers developing treatments for psychiatric and neurological diseases. But now, a partnership that combines Calyx’s clinical trial imaging expertise and Ceretype’s novel functional MRI (fMRI) platform enables trial sponsors to overcome this challenge and see the physical action of a drug on patients with psychiatric and other neurological disorders.
Topics Covered: Medical Imaging

This session will focus on the difficulty of evaluating HCC patient scans after local intervention and how sponsors can overcome the complexities associated with imaging in HCC clinical trials.
Topics Covered: Medical Imaging

In this webinar, Calyx’s Peter Tarbox shares the RTSM factors oncology trial sponsors should consider in their IRT design, focusing on how advanced IRT systems can accommodate changes in centrally and locally sourced medication for optimal clinical trial supply management.
Topics Covered: IRT

In this webinar, Calyx’s Malcolm Morrissey and Maarit Kelvin Korpos review other scenarios of how study data can lead to unintentional unblinding and the processes sponsors and their IRT solution providers should follow to mitigate these risks.
Topics Covered: IRT

Get a glimpse into what it’s like to work with Calyx IRT and how we customize our configurable core IRT system to meet your specific study needs.
Topics Covered: IRT

This scientific discussion will review how emerging AI solutions that employ quantitative neuroimaging can help address some of the key challenges faced in Alzheimer’s Disease.
Topics Covered: Medical Imaging

In this webinar, Calyx’s Head of Statistics and Product Support Services, Malcolm Morrissey shares his experience on how to identify the randomization implementation options that can lead to unblinding of the block size and other potential risks that could result in selection bias at the site.
Topics Covered: IRT

In this webinar, Dr. Oliver Bohnsack of Calyx – a leading expert on RECIST and irRECIST – discusses the implications of these findings, what it means for oncology clinical development and treatment decision-making, and why irRECIST easily can and shall replace outdated RECIST 1.1 on all solid tumor trials going forward.
Topics Covered: IRT

A panel of clinical trial sponsor and distribution partner representatives – all with experience in direct-to-patient shipments – will review what is possible to achieve and what constraints may be encountered when considering DtP for clinical trials. We’ll also hear from the most important clinical trial stakeholder, a patient who has experienced the shift from in-clinic visits to treatment delivered at their doorstep; he will share his perspective as it relates to the development of a DtP clinical trial strategy.
Topics Covered: DtP

Topics Covered: DtP


Topics Covered: Medical Imaging

In this live virtual event, a panel of key opinion leaders including IMWG 2016 authors and imaging experts will answer your questions about the changing landscape of imaging in multiple myeloma trials and how to optimize imaging-related assessments.
Topics Covered: Medical Imaging

In this webinar we review how, by applying advanced IRT settings to control the supply chain, medication can be directed to recruiting sites only and amounts shipped to those sites can be reduced to the minimum, overall limiting the quantity of medication wasted at site.
Topics Covered: IRT

Join Calyx’s Head of Statistics and Product Support Services, Malcolm Morrissey, for a webinar focusing on an undervalued aspect of study and IRT design.
Topics Covered: IRT
To: Calyx
From: Top 5 Pharmaceutical Company
Subject: Calyx Medical Imaging helps get patients treated quickly
A very big thank you to Calyx Medical Imaging who worked outside of their turnaround timelines to review the scans that ultimately allowed the central team to approve enrollment to facilitate treatment of this patient as quickly as possible. This is a true testament of our values in ensuring that we get treatment as quickly as possible to patients that are in need.

To: Calyx
From: Top 5 Pharmaceutical Company
Subject: IRT team are great collaborators
I’m very happy with the collaboration we have seen with Calyx’s IRT team, their open communication, and their ability to discuss and solve issues.

To: Calyx
From: Top 5 Pharmaceutical Company
Subject: Calyx Medical Imaging Made this Approval Possible
A big thank you to the Calyx Medical Imaging team. This (approval) is also your accomplishment, and you should be very proud of the work you have done to make this possible. More approvals to come!
– Global Trial Manager
Liver Cancer Development Program

TO: Calyx
FROM: Veralox
SUBJECT: Calyx Medical Imaging – Thank you for meeting tight timelines
I am very impressed with the Calyx Medical Imaging team who have been incredible collaborators in helping us to advance our lead program. We especially appreciate the team working on tight timelines to help us meet our study start initiation goals. Given that we are a small company, we rely on our collaborators, and the team at Calyx works seamlessly beside us to help us advance our development programs. I look forward to continuing our partnership!
– Alicia Herr, PMP,
Veralox Therapeutics

To: Calyx
From: Top 5 Pharmaceutical Company
Subject: Calyx Medical Imaging – Transparent, Constant Collaboration
We worked very closely with Calyx on the pivotal uterine fibroid studies. Critical components of success included transparent constant collaboration with real-time communication at all levels between our study team and Calyx. This kind of communication throughout the life of the study helped to ensure our team had the information needed to make timely informed decisions and to deliver quality data for our deliverables. We were able to establish lessons learned from prior studies and implement those on this study. I’m looking forward to continuing our work together as we strive to bring more treatment options to women.

To: Calyx
From: Arrivent
Subject: Global Oncology Program – Calyx Medical Imaging Head and Shoulders above the Rest
I am writing to give testimony to the expert independent central imaging review services provided by Calyx in support of two of ArriVent’s global clinical oncology studies. Calyx has deep technical expertise in various tumor response criteria, imaging modalities, image collection logistics, data management and regulatory requirements. They gave ArriVent invaluable advice during protocol and charter development. This is coupled with a team of world-class highly specialized independent radiologists, imaging scientists, project managers, medical writers, a very efficient budget and contract group as well as superb internal processes and systems all of which contributed to a very smooth program kick-off for our lead molecule.
We have interviewed a number of different imaging CROs and Calyx is definitely head and shoulder above the others.
– Morgan Lam
SVP, Development Operations & Business Management,
Arrivent Biopharma

To: Calyx
From: Top 3 Pharmaceutical Company
Subject: Thank you Calyx Medical Imaging – Osteoporosis Trial Success
I would like to thank the Calyx Team very much for your contribution to this wonderful study outcome. Without you this marketing authorization would have never been possible.
-Medical Lead, Osteoporotic Trial,
Top 3 Pharmaceutical Company

To: Calyx
From: ProTrials
Subject: Pleased to partner with Calyx
We’re pleased to partner with Calyx and are confident that our customers will benefit from the scientific, medical, and clinical expertise they have honed during their 30 years of delivering reliable eClinical solutions to the clinical development industry.
– Christy Meyer
Director, Quality Insurance,

To: Calyx
From: LG Chem
Subject: Calyx IRT – Simplifying Complicated Randomization
I sincerely appreciate your cooperation and Calyx’s team effort for our program.
We could not have successfully completed the IRT development for both Ph3 studies without Calyx’s team expertise.
As you know, our Ph3 studies are very complex and your high degree of experience, knowledge and system (platform) were required in order to develop the IRT we wanted. Also, there were some challenges and concerns due to protocol amendment by LGC during IRT development.
But, we relied heavily on Calyx’s expertise in IRT to deliver these two complex trials in our Gout program. Calyx made recommendations on how to overcome some of the challenged we were facing, and it resulted in a solution that perfectly meets our needs.
I have been working for 13 years in clinical development area in pharmaceutical industry. Even if my experience was relatively short, I can tell that Calyx’s platform is very valid and elaborate compared to other IRT systems.
Thank you so much.
– Younghwan Jang,
Study Lead,
LG Chem, Ltd.

To: Calyx
From: ClinChoice
Subject: Partnering with Calyx for reliable data outcomes
We chose to partner with Calyx due to their tenured scientific, medical, and technical teams who possess a depth and diversity of experience in providing reliable data outcomes.
– Tiepu Liu, President,
Global Biometrics,
ClinChoice

test

