Five Key Considerations
As the industry turns toward data-centricity, optimizing the management of your regulatory submissions and product data maintenance becomes ever more critical. Investment in strategic regulatory initiatives and Regulatory Information Management (RIM) capabilities continues to be a priority across the industry.
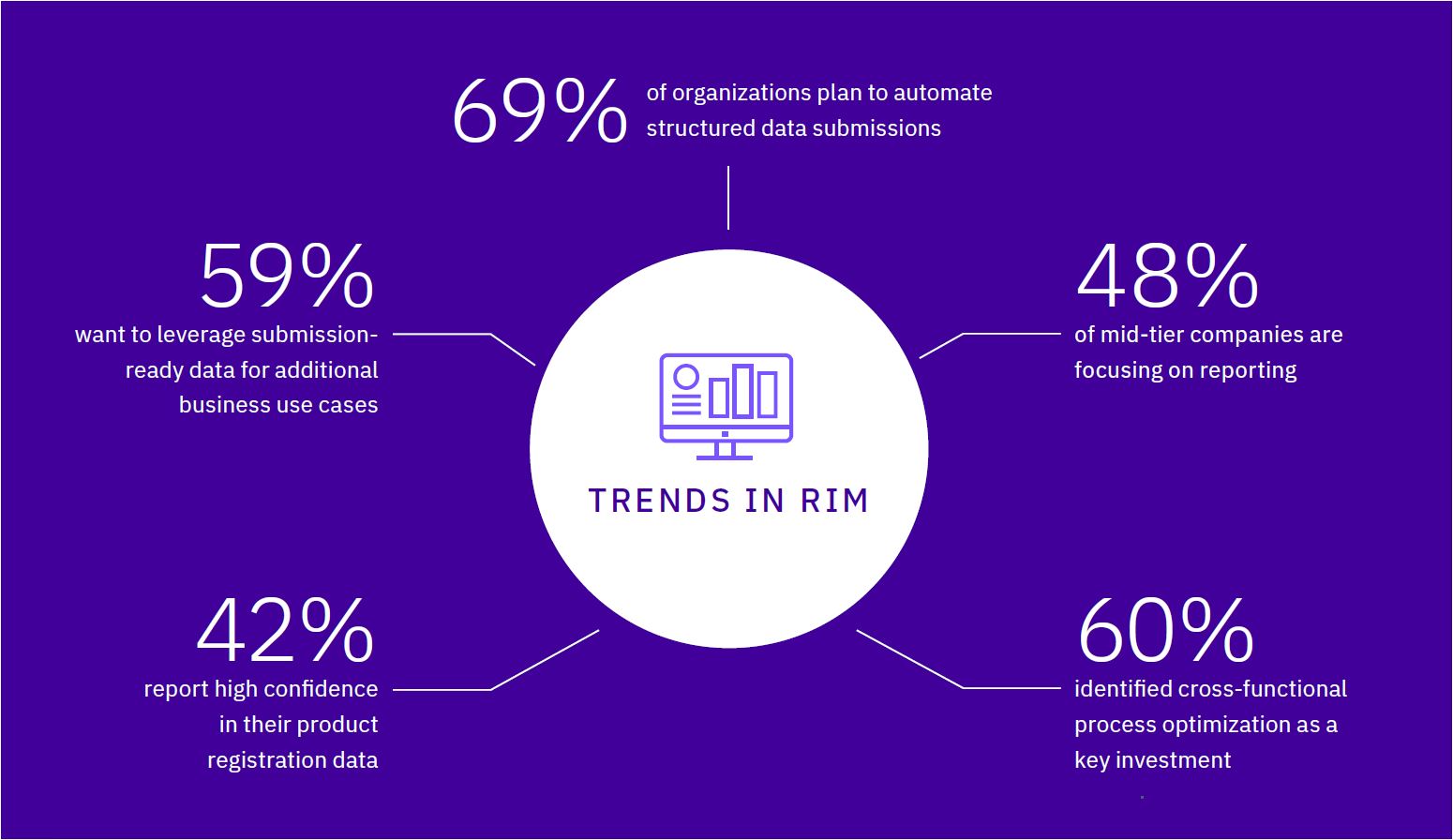
Five Considerations for Adopting RIM

1. DATA QUALITY SUSTAINABILITY
Data governance, compliant regulatory submissions, extensive data sets for XEVMPD/IDMP compliance.
Use your RIM system for onward sustainability of these to manage your data and publish your structured data submissions.
Aligning with your day-to-day regulatory processes promotes sustainable data.

2. OPTIMIZED PROCESSES
Optimized business processes are critical for a global regulatory team to operate effectively in today’s fast-evolving environment.
Harmonized processes across corporate and subsidiary operating sites:
- Optimize resources
- Reduce cycle time
- Increase data quality
- Ensure submission compliance
- Decrease data entry burden

3. REALIZED BUSINESS BENEFITS
Drive optimal performance by laying the foundation of your organizational strategy.
Process and system maturity promote trust in your data. Leverage that data to make informed business decisions.
Maximize your business benefits via strong data visualization capabilities in your RIM platform.

4. ORGANIZATIONAL COMPETENCY
What is the outcome of organizational competency?
- Harmonization
- Standardization
- Collaboration
- Eliminated waste
- Reduced costs
- Accelerated product release
The bottom line? More effective operations drive more competitive advantages in global markets.
5. CONNECTIVITY
Make the most of your best-of-breed systems throughout the organization.
Establish a source of truth and eliminate the distraction of extensive change management programs. The touch points from your RIM data to the rest of the organization make this paramount.
HOW TOP PERFORMERS ARE CONNECTED
Top-performing organizations are eight times more likely to have established a connection between their RIM system and Safety, and three times more likely to have established a RIM connection with product supply and product change control processes, compared to their counterparts.
What Can You Expect?
A modern and compliant system, Calyx RIM delivers:
- Harmonized data and compliant, high-quality submissions
- Expedited time-to-market resulting from improved processes
- Operational and cost efficiencies
- More focus on core competencies by regulatory resources
THE ABILITY TO TRACK, MINE AND ANALYZE DATA WITHIN YOUR RIM SYSTEM IS CRITICAL TO REGULATORY COMPLIANCE AND, ULTIMATELY, TO PATIENT SAFETY.
A Trusted Partner
Calyx uniquely combines regulatory cloud technology with the depth and breadth of our regulatory expertise to deliver a scalable and flexible RIM solution that yields predictable year-over-year savings and addresses regulatory complexities, increased workloads, and limited budgets facing the industry.