Industry-proven clinical trial management
Your clinical trials deserve every chance to succeed. And your trial monitors deserve the most effective tools to improve trial efficiencies. Calyx CTMS is the solution.
Since 1992, Calyx has pioneered the field of clinical trial management, delivering an effective CTMS that improves trial efficiencies and drives successful clinical development programs.
Worldwide biopharmaceutical companies and CROs repeatedly turn to Calyx to meet their CTMS needs.
Optimized through the delivery of 60,000+ trials, Calyx CTMS can adapt to meet the complexity or simplicity of your clinical trials and processes.
Join the world’s top 20 pharmaceutical companies and CROs who rely on Calyx CTMS and leverage its industry-proven features to:
- Reduce trial risks, ensure compliance
- Optimize the future, learn from trial performance
- Improve data quality and boost productivity
Reduce trial risks, ensure compliance
With Calyx CTMS, you leverage robust functionalities that reduce trial risks and ensure compliance at multiple levels.
Key Benefits
- Avoid study delays and/or increased study costs by tracking planned vs actual recruitment and enrollment so that correction plans can be implemented in a timely manner.
- Complete your start-up processes faster by utilizing study-specific templates.
- Monitor planned vs actual site initiation and enrollment and pivot accordingly.
- Help your monitors focus on the right tasks by providing key site information and customizable checklists.
- Ensure compliance of monitoring visit reports using end-to-end workflows with electronic signatures for creation, review, and approval.
- Ensure financial compliance and payment control through fund and payment authorization workflows. Streamline planning and management of site payments.
- Ensure compliance regarding expiring documents with proactive notifications.
Optimize the future, learn from trial performance
Make informed decisions to improve your trial’s success through efficient cross-study analysis. Calyx CTMS enables you to centralize the operational data of all your historical trials into a single source – removing the need for separate data sources – so that you can learn from past trial outcomes to optimize current and future trials.
Key Benefits
- Identify emerging trends in your current trials. Calyx CTMS has been used to analyze the COVID-19 pandemic and observe, in real-time, drops in site visits and increases in remote visits, country by country.
- Get inspiration from previous successful trials to build realistic plans for your next trials.
- Select the optimal sites to increase your study’s chance of meeting timelines (subject recruitment challenges, start-up). Select qualified investigators by monitoring experience and performance metrics.
Improve data quality and boost productivity
Rely on Calyx CTMS as the heart of your clinical system and the source of truth for all your operational data including information about the study, country, site, personnel, vendor, etc. Seamlessly connecting your CTMS to the rest of your ecosystem is critical to ensuring good data quality (and avoiding discrepancies between systems), ultimately boosting the end user’s productivity.
Leverage our expertise in integrating Calyx CTMS with your other eClinical systems. You will be able to configure and activate our standard integrations, as well as collaborate on custom integrations.
Key Benefits
Use our standard integrations with top EDC, IRT, or eTMF systems:
- Avoid painful double data entry and realize real-time recruitment information from your EDC system.
- Help ensure you’re inspection-ready with instant access to monitoring visit reports through integration with your eTMF systems.
Leverage our extensive experience to build custom integrations with your key systems.
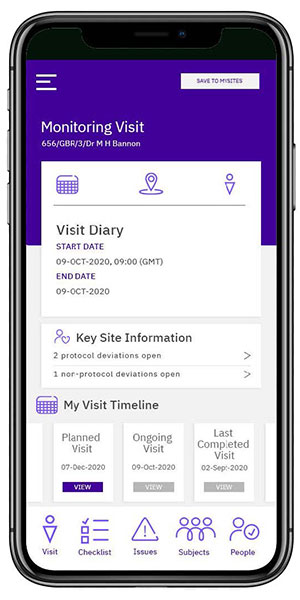
Change the way monitors work
The Calyx CTMS mobile application empowers monitors to manage clinical site data anytime, anywhere.
- No internet? No problem!
- Quick and easy access to site and subject information
- Navigation is simple and intuitive
- Risks are immediately identified, allowing for faster resolution
- Monitoring tasks are completed quickly and efficiently
- Extensive note taking is eliminated and data discrepancies are reduced
- Instant communication with clinical site staff
- Integrates with phone, mapping, Outlook apps, and Calyx CTMS
- For use with iPhone and Android