Your Images. In Safe Hands.
Managing an imaging trial is more than site qualification, QC, and reads. Without experience and scientific expertise backed by hundreds of regulatory approvals, an imaging provider’s long-term value is transactional at best, and impossible at worst.
Since 1997, Calyx has helped global biopharmaceutical companies and CROs successfully leverage medical imaging in their clinical trials. We offer the full range of imaging services to enable transparent and standardized processes and deliver therapeutically aligned value that allows you to best assess the safety, efficacy and effectiveness of your compounds.

With Calyx, you can rest assured that your endpoint data will be derived with quality and per current regulatory guidelines.
Scientific Expertise
Choosing an imaging partner with experience in successfully implementing and delivering complex medical imaging trials is critical when your study endpoints are at stake. At Calyx, we devote dedicated, on-staff scientific expertise to support your study from start to finish. This includes proven processes in acquisition guideline development and site training to charter development, criteria implementation, reviewer training, and data monitoring.
Calyx’s scientific and medical team of 75 full-time imaging experts are here to ensure that we design and deploy the right solutions specifically tailored to meet your individual research requirements. And you can have confidence in our reviewer network, which includes over 1,100 board-certified readers, made up of industry leaders, criteria authors, and radiology
department chairs.
Image Management
At Calyx we’ve instituted a culture of “No image left behind.” Our process for quality control and reads standardizes and normalizes image data during the course of a trial, enabling us to consistently meet two-day turnaround times for image feedback. As a result, you receive results faster to receive critical go/no-go decisions, and your sites receive feedback sooner to help them improve efficiencies.
Project Management
Operationally, Calyx’s project leadership staff are trained to offer mature and consultative management of your trial to make actions clear and ensure milestones are achieved.
Our medical imaging experience is drawn from managing over 2,600 studies to date which include approximately 3.9 million images from sites in over 80 countries.
Why trust anyone else with your imaging study?
2,600
STUDIES
3.9 million
IMAGES
80
COUNTRIES
Therapeutic Experience
No two studies are alike. Leverage Calyx’s broad experience to ensure your imaging needs are met and your trial is optimally supported.
ONCOLOGY
Cervical
CLL
Colorectal
Gastric Carcinoma
GIST
Glioma
Head and Neck
Hepatocellular
Leiomyosarcoma/Liposarcoma
Lymphoma
Lung
Melanoma
Ovarian
Pancreatic
Pelvis
Prostate
Renal Cell
Thyroid
Urothelial
NEUROLOGY
Dementia
Epilepsy
Migraines
Multiple Sclerosis
Parkinson’s
Seizure
Stroke
MUSCULOSKELETAL
Bone Age
Bone Density
Bone Repair/Healing
Osteoarthritis
Osteogenesis Imperfecta
Osteoporosis
Psoriatic Arthritis
Rheumatoid Arthritis
CARDIOVASCULAR
Coronary Artery Disease
Ischemic Heart Disease
Peripheral Vascular Disease
Myocardial Infarct
Left Ventricular Dysfunction
Deep Vein Thrombosis
+ THERAPEUTIC AREAS
COPD
Dermatology
Diabetes
Diagnostic Agents
DVT
Erectile Dysfunction
Goiter
Hepatitis C
HIV
IPF
Lipodystrophy
Muscle Wasting
Myelofibrosis
Obesity
Pneumonia
Polycystic Kidney Disease
Sinusitis
Endometriosis
Irritable Bowel Syndrome
Atopic Dermatitis
Imaging Done Right
It takes more than just technology to manage an imaging trial well and deliver data that visualizes safety, efficacy and effectiveness. Calyx delivers deep-rooted scientific expertise to plan and govern, a tested process of handling data efficiently and with limited site burden, and well-designed systems to help your trial succeed.
Comprehensive Services
As the leading imaging provider, we offer a full range of services, including:
– Consultation
– Standardized image acquisition & collection
– Image processing & analysis
– Regulatory submission support
– Image warehouse & DICOM exportability
Calyx Delivers Technology-enabled Services throughout Clinical Development
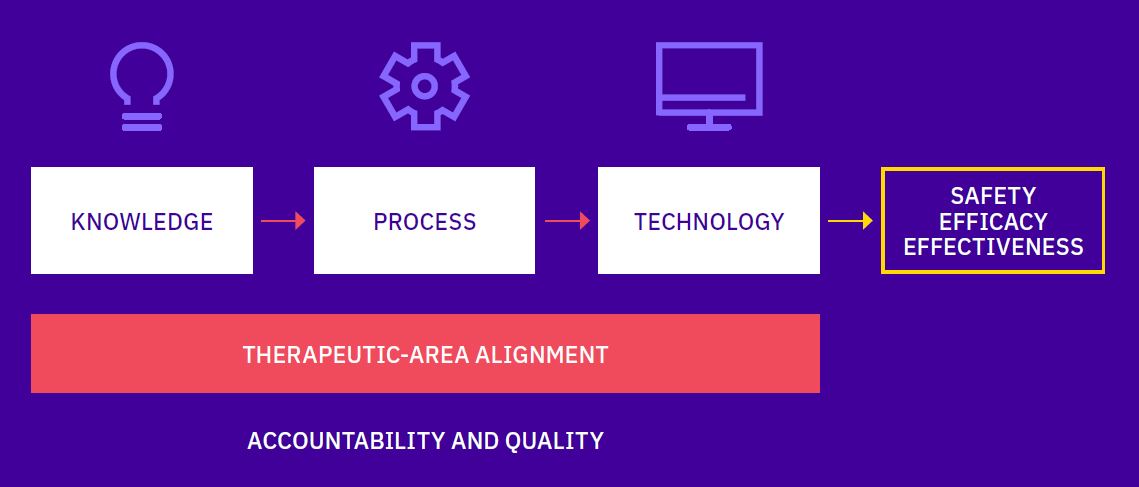
Image Lifecycle Support
Get from protocol to submission. Faster.
Reliable Reads. Every Time.
Your images deserve to be evaluated by the best minds.
Calyx’s approach to medical imaging provides flexibility, scalability and rapid deployment while ensuring the highest quality, every time. Image review personnel undergo rigorous training and testing to deliver high-quality analysis data based on independent and unbiased image evaluations.
Our radiologists are the most esteemed and respected physicians in the world. As active practitioners, they have a current understanding of the disease and its impact on patients.
Our use of smart technology — i.e., auto-population of measurements into CRFs and edit checks on criteria rules — removes noise and allows them to do what they do best: analyze your medical images.
Located throughout North America, Europe, China and India, our readers have experience in the unique requirements of performing blinded independent reads for clinical trials. We partner with world-class hospitals and have established on-site labs, enabling radiologists to read within their clinical schedule or from home workstations for complete flexibility.

With industry-leading readers and proven approach to clinical trial imaging, you gain confidence that your compound will pass regulatory scrutiny.
Flexible Imaging Services
Trust Calyx to support your imaging study, with services geared toward your varying needs, including:
Calyx’s approach to medical imaging provides flexibility, scalability and rapid deployment while ensuring the highest quality, every time. Image review personnel undergo rigorous training and testing to deliver high-quality analysis data based on independent and unbiased image evaluations.
Our radiologists are the most esteemed and respected physicians in the world. As active practitioners, they have a current understanding of the disease and its impact on patients.
Our use of smart technology — i.e., auto-population of measurements into CRFs and edit checks on criteria rules — removes noise and allows them to do what they do best: analyze your medical images.
Located throughout North America, Europe, China and India, our readers have experience in the unique requirements of performing blinded independent reads for clinical trials. We partner with world-class hospitals and have established on-site labs, enabling radiologists to read within their clinical schedule or from home workstations for complete flexibility.
FULL SERVICE
We train your sites, receive and QC your images, author charters and all critical documents, engage and have images read by expert independent radiologists, and provide fully validated data exports with medical QC and outlier review built in.
EXPLORATORY / EARLY PHASE STUDIES
Scalable, “Follow the molecule” solutions
COLLECT AND HOLD
Balance risk and budget by waiting to have images read until you are sure you need the data and when that signal is known. Ensure that you’re not late to market because we have your images, ready to read, and ready for you to provide data to regulatory agencies.
RETROSPECTIVE / RESCUE
We tell the truth, lay out the risks, and help you make the hardest decisions to support your ultimate goal of moving drug development forward. We have supported hundreds of retrospective and rescue trials.
BREAKTHROUGH
We’ve guided over 70 Breakthrough Therapy trials. In fact, Calyx Imaging supported the majority of Breakthrough Therapy approvals granted by the FDA in 2019/20. Our imaging experts have seen it all and can share an insider’s view of what happens when the stakes, scrutiny, and demand are all sky-high.
CONSULTING
Have you ever struggled to choose the right imaging biomarker for a given mode of action, indication, and study phase? Have you ever needed help writing sections of your protocol? Or imaging biomarker performance data to adequately power your study?
Calyx’s subject matter experts can support you with customized workshops or become an extension of your clinical team to accelerate the development of your program. If we don’t have the knowledge inhouse, we can connect you with external key opinion leaders who do.
Global, Yet Local.
As clinical trials become increasingly complex and large-scale, involving many different regions around the world, it’s critical to partner with someone whose global capabilities and infrastructure you can trust.
At Calyx, our people understand your local challenges and can support your study teams and sites in local languages. With dedicated imaging teams located around the world including offices in USA, England, Berlin, China, Japan, and India, we provide truly global and seamless deployment. Our global footprint means “around-the-clock” processing, quality control, site management and real-time support. As a result, we adhere to a uniform, rigorous methodology to assure consistent processes and operating procedures regardless of location but ensuring cultural consciousness for each local market.
Patients Matter
While Calyx’s people, processes and technology allow us to boast about first-time quality, 48-hour turnaround times, and regulatory compliance, we are most proud about the impact our imaging solution and services have on the lives of enrolled patients.
With every efficiency, quality control, and remediation we introduce into the process, we improve the clinical trial experience, increase quality of outcomes data, and reduce the time it takes for you to get new therapies to patients who need them.
Learn how Calyx can reliably solve your most complex clinical trial imaging challenges and help you get treatments to market sooner.