Calyx CTMS is a leading enterprise solution to plan, administer and track every aspect of global clinical trials from start-up to closeout. A centralized system to orchestrate all operational and administrative activities throughout the clinical hierarchy, it allows biopharmaceutical sponsors and clinical research organizations (CROs) to intelligently manage the complexities of clinical trials by simplifying workflows and proactively managing risks across the trial lifecycle. Containing all operational trial data, the solution allows consistent and accurate information to be shared across the entire enterprise, thereby facilitating timely and informed decision-making.
Brochure

CTMS Tracking & Management
CTMS tracking and management handles the planning and tracking of all projects and trials, providing ongoing visibility on the health of clinical programs to enable robust operational oversight and transparency. This includes participating countries and sites, to details of the patients and their progress. It holds all information relating to the trials such as milestones, visit designs, timelines, enrollment and site activation metrics as well as detailed issue/contact tracking.

CTMS Monitoring
The site monitoring functionality is an online/offline web-based tool that supports all aspects of site management and monitoring, including collection of site monitoring data, milestone planning, protocol deviations, issues and subject recruitment. Data collected automatically populates the monitoring visit report, with the entire review and approval process being managed within the CTMS system. Calyx CTMS also supports a secure, unblinded monitoring capability suitable for independent drug monitoring or other monitoring functions performed by an unblinded monitor. Available on laptops and tablets, it provides optimal system portability and flexibility for site management and monitoring.

CTMS Monitoring Mobile App
The monitoring mobile app is an exciting new solution that allows site monitors to work offline using their iPhone or Android smartphones. The app empowers site monitors to manage clinical site data anytime with simple and intuitive navigation. Users can access site and subject information, start a monitoring visit, capture and review issues for faster resolution. The App supports contemporaneous and efficient completion of monitoring tasks while eliminating extensive note-taking and data discrepancies. In addition, it integrates with Outlook email and calendar for optimal flexibility.

CTMS Clinical Cost Tracking
The clinical cost tracking manages the planning, projection and tracking of costs associated with clinical trials by employing multi-center, multi-currency and full-cost analysis functionality. With a sophisticated and automated investigator payment facility which can be integrated with corporate accounting systems, it provides a full payment generation, review and approval process, facilitating financial management of hundreds of sites at a time.

CTMS Investigator
The investigator functionality provides a profile of investigator data and enables selection of the best investigators for future studies. Dynamic lists can be created to be shared, refined and reviewed for site feasibility. The functionality helps maximize the value of historical data with analysis of investigator performance, experience and expertise, lending valuable decision support for investigator and site selection.

CTMS Regulatory Document Tracking
The regulatory document tracking streamlines the onerous and challenging task of ensuring timely and complete regulatory documentation for the study. The functionality focuses on collecting the right documents during study start-up to get investigative sites approved and activated on time. It can be adapted on a trial-bytrial and country-by-country basis to ensure the correct documents are gathered, whether an FDA 1572, an informed consent approval or a protocol signature page.

CTMS Task Management
The task management functionality helps focus and prioritize user attention on those activities requiring attention based on date or status. CTMS is a large system with users often supporting multiple sites across studies. Instead of users running reports or unfocusedly browsing the system, the functionality presents a realtime, dynamic “to do” list of milestones, site visits, and other events. Users can drill into the record to make necessary updates, return to the task management screen, and select the next record requiring their attention.
Industry-proven clinical trial management
Your clinical trials deserve every chance to succeed. And your trial monitors deserve the most effective tools to improve trial efficiencies. Calyx CTMS is the solution.
Since 1992, Calyx has pioneered the field of clinical trial management, delivering an effective CTMS that improves trial efficiencies and drives successful clinical development programs.
Worldwide biopharmaceutical companies and CROs repeatedly turn to Calyx to meet their CTMS needs.
Optimized through the delivery of 60,000+ trials, Calyx CTMS can adapt to meet the complexity or simplicity of your clinical trials and processes.
Join the world’s top 20 pharmaceutical companies and CROs who rely on Calyx CTMS and leverage its industry-proven features to:
- Reduce trial risks, ensure compliance
- Optimize the future, learn from trial performance
- Improve data quality and boost productivity
Reduce trial risks, ensure compliance
With Calyx CTMS, you leverage robust functionalities that reduce trial risks and ensure compliance at multiple levels.
Key Benefits
- Avoid study delays and/or increased study costs by tracking planned vs actual recruitment and enrollment so that correction plans can be implemented in a timely manner.
- Complete your start-up processes faster by utilizing study-specific templates.
- Monitor planned vs actual site initiation and enrollment and pivot accordingly.
- Help your monitors focus on the right tasks by providing key site information and customizable checklists.
- Ensure compliance of monitoring visit reports using end-to-end workflows with electronic signatures for creation, review, and approval.
- Ensure financial compliance and payment control through fund and payment authorization workflows. Streamline planning and management of site payments.
- Ensure compliance regarding expiring documents with proactive notifications.
Optimize the future, learn from trial performance
Make informed decisions to improve your trial’s success through efficient cross-study analysis. Calyx CTMS enables you to centralize the operational data of all your historical trials into a single source – removing the need for separate data sources – so that you can learn from past trial outcomes to optimize current and future trials.
Key Benefits
- Identify emerging trends in your current trials. Calyx CTMS has been used to analyze the COVID-19 pandemic and observe, in real-time, drops in site visits and increases in remote visits, country by country.
- Get inspiration from previous successful trials to build realistic plans for your next trials.
- Select the optimal sites to increase your study’s chance of meeting timelines (subject recruitment challenges, start-up). Select qualified investigators by monitoring experience and performance metrics.
Improve data quality and boost productivity
Rely on Calyx CTMS as the heart of your clinical system and the source of truth for all your operational data including information about the study, country, site, personnel, vendor, etc. Seamlessly connecting your CTMS to the rest of your ecosystem is critical to ensuring good data quality (and avoiding discrepancies between systems), ultimately boosting the end user’s productivity.
Leverage our expertise in integrating Calyx CTMS with your other eClinical systems. You will be able to configure and activate our standard integrations, as well as collaborate on custom integrations.
Key Benefits
Use our standard integrations with top EDC, IRT, or eTMF systems:
- Avoid painful double data entry and realize real-time recruitment information from your EDC system.
- Help ensure you’re inspection-ready with instant access to monitoring visit reports through integration with your eTMF systems.
Leverage our extensive experience to build custom integrations with your key systems.
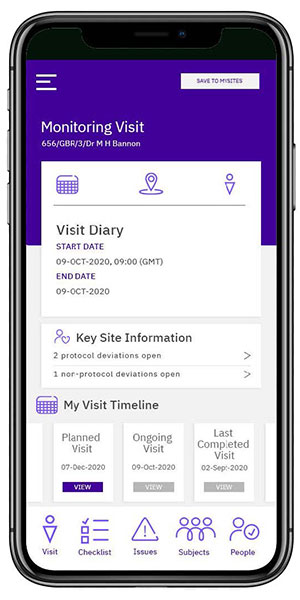
Change the way monitors work
The Calyx CTMS mobile application empowers monitors to manage clinical site data anytime, anywhere.
- No internet? No problem!
- Quick and easy access to site and subject information
- Navigation is simple and intuitive
- Risks are immediately identified, allowing for faster resolution
- Monitoring tasks are completed quickly and efficiently
- Extensive note taking is eliminated and data discrepancies are reduced
- Instant communication with clinical site staff
- Integrates with phone, mapping, Outlook apps, and Calyx CTMS
- For use with iPhone and Android
This case study reviews how a leading pharmaceutical company with more than 30 development projects in clinical trials worldwide is relying on Calyx CTMS to make robust, data-driven decisions that ensure patient safety, increase the efficiency of its business processes, and contain costs – all of which enables timely trial completion and license filings.
Case Study












